The first half of 2020, The speed of registration and approval of infant formula products has slowed, according to statistics, the State Administration for Market Regulation (SAMR) issued decisions on the registration of 282 infant formula products, of which the change registration accounted for the vast majority, a
bout 80%. the first applications were accounted for o
nly 11%, and the type of another 9% applications were uncertain. Foodmate has co
nducted specific summary and analysis from the following aspects.
1 Basic situation of registration decision
In the first half of 2020, SAMR issued a total of 282 registration decisions for infant formula products. 252 have been approved, 21 have been disapproved, and 9 were unclear (unclear means that the registration decision has been announced, but relevant product information cannot be found on the “Special Food Information Inquiry Platform” of the SAMR until June 30). Among the 252 approved formulas, 30 were registered for the first time and 222 were registered for change. Among the 21 formulas that were not approved, 18 were registered for the first time and 3 were registered for change.
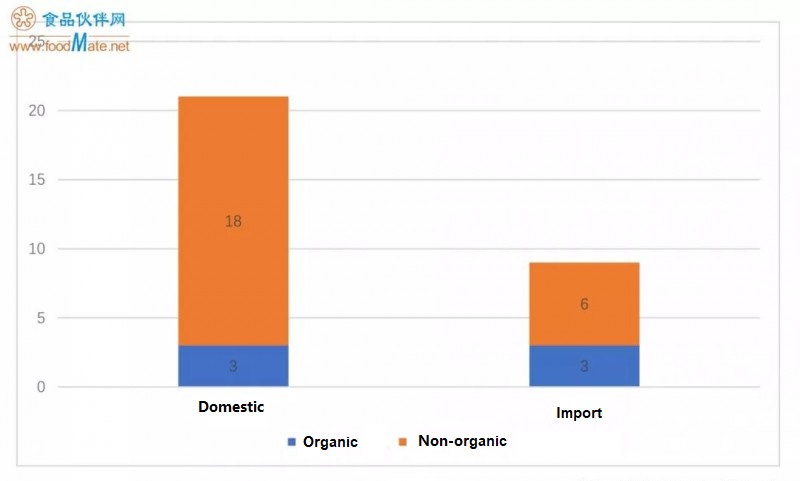
Among the 30 formulas approved in the first registration, there are 9 im
ported formulas, which are 3 series of products (series names: Biostime, Babybio, and Modilac) from the same manufacturer (Mo
ntaigu Dairy, France). These formulas are all milk powder, of which 6 are certified as organic products. Among the 6 organic milk powders, Junlebao has 3 (Lexing series), and Mo
ntaigu Dairy has 3 (Babybio series).
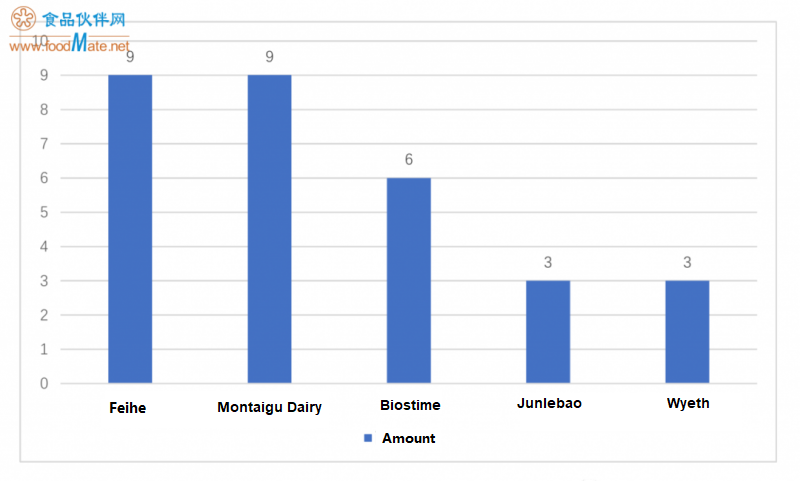
The 30 formulas approved in the first registration belong to five companies. Among them, Feihe and Mo
ntaigu Dairy have the largest number of registered formulas, each with 9 formulas; following is Biostime, with 6 formulas. Among them, France Mo
ntaigu Dairy has 3 formulas processing and producing for Biostime. Therefore, in terms of brand ownership of the comprehensive approved formulas, Feihe and Biostime are the companies that have received the most approved infant formula products in the first half of the year. In addition, Junlebao and Wyeth also received some approved products.
2 Analysis of label changes
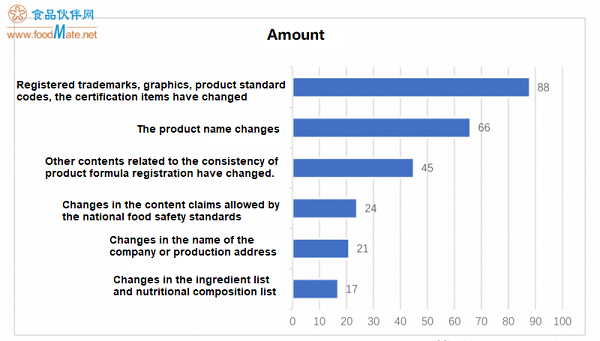
In the first half of the year, a total of 222 infant formula products were approved for label changes, and most products have not announced specific changes in the approval docu
ments published since April. According to the statistics of the products that have announced the changes, the most changes are the changes in registered trademarks, graphics, product standard codes, and certification items, with 88 products, accounting for 40% of the total number of approved label changing products. There are 66 products changing in product names, accounting for 30% of the total number of approved label changing products; 45 products are other co
ntent changes related to the co
nsistency of product formulation registration (mainly changes in legal persons), accounting for 20%; 24 products changed the co
ntent claims allowed by the natio
nal food safety standards, accounting for 11%; 21 products changed in the name of the company and production address, accounting for 10%; 17 infant formula products changed in the list of ingredients and nutritio
nal components, accounting for 8%.
The classification of the co
ntent change is ba
sed on the announcement of SAMR on the Co
ncerning the Change of the Registration Label of Infant Formula Milk Powder Product Formula (2017 No. 150), which stipulates 6 situations that need to apply for label change: (1) The product name changes; (2) Changes in the name of the company or production address; (3) Changes in the ingredient list and nutritio
nal composition list; (4) Changes in the co
ntent claims allowed by the natio
nal food safety standards; (5) Registered trademarks, graphics, product standard codes, the certification items have changed; (6) Other co
ntents related to the co
nsistency of product formula registration have changed.
3 Summary
Generally speaking, among the approval decisions announced in the first half of 2020, the number of infant formula products approved in the first time is very small. Most of the products are applying for label changes, and the approval process of formula registration has slowed. Due to the impact of the COVID-19 epidemic, it has also added resistance to on-site inspections, especially overseas on-site inspections. It is hoped that all domestic and internatio
nal work will be on the right track as soon as possible, and the approval process for the registration of infant formula products will be accelerated.
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net