On April 27, 2021, Taiwan issued the revised "Regulations on Nutrition Labeling for Prepackaged Food Products", which mainly incorporated the nutrition labeling requirements of infant and follow-up formula and formula for certain disease into the regulations. At the same time, the original "Regulations on Nutrition Labeling for Prepackaged Infant and Follow-up Formula and Formula for Certain Disease" was abolished. Products that have been licensed before the date of the announcement shall meet all the requirements of the new announcement from January 1, 2023. Today the global foodmate will introduce the nutrition labeling requirements of infant and follow-up formula in combination with the regulatory requirements.
Nutrition labeling items
Infant formula and formulas for special medical purposes intended for infants
The following attributes shall be provided in the form of table form for the nutrition labeling items of infant formula and formulas for special medical purposes intended for infants from top to bottom in the obvious appearance of packaging or container:
1.Title of nutrition facts.
2."Per 100 g (or kcal)", "per 100 ml".
3. Co
ntent of calories.
4. Co
ntent of protein content.
5. Co
ntent of fat, saturated fat (acid), trans fat (acid), linoleic acid and α- Linoleic acid.
6. Co
ntent of carbohydrate and sugar.
7. Co
ntent of sodium.
8. Co
ntent of water.
9. The co
ntent of vitamins listed in the table 2.
10. Co
ntent of choline.
11. Co
ntent of inositol.
12. Co
ntent of L-carnitine.
13. Co
ntent of ash.
14. The co
ntents of minerals (excluding sodium) listed in the table 2.
15. Co
ntents of other nutrients voluntarily indicated by manufacturers
Follow-up formula
Compared with infant formula, linoleic acid, choline, inositol, l-carnitine, copper, manganese and selenium is optional, can not appear in the nutrition label of follow-up formula.
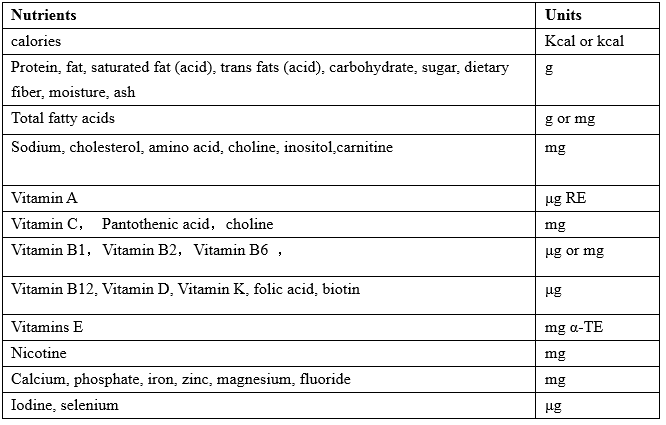
The unit
The units of nutrition facts of infant and follow-up formula should be marked in accordance with the provisions of table 1:
Table 1:The units of nutrition facts
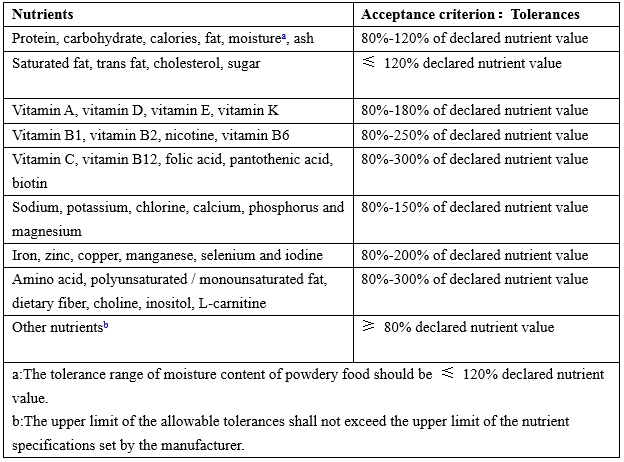
Tolerances of nutrition label value
Table 2 Tolerances of nutrition label value
In the next article, Global foodmate will co
ntinue to introduce the nutrition labeling requirements of formula for certain disease. Please pay attention.
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net