![[230612]a4f1761ad8f41f278308e4e523dfc5dc.jpeg.jpeg [230612]a4f1761ad8f41f278308e4e523dfc5dc.jpeg.jpeg](https://global.foodmate.net/file/upload/image/20230612/[230612]a4f1761ad8f41f278308e4e523dfc5dc.jpeg.jpeg)
The European Commission recently published Regulation (EU) 2023/915 and repealed Regulation (EC) No 1881/2006. The new regulation came into effect on 25 May, 2023. This regulation adjusts the limits for contaminants in food, including formula foods for infant.
In order to ensure the safety and nutritional adequacy of formula foods for infant and young children, it is necessary for enterprises to refer to a large number of scientific evidence for product development. This includes relevant regulations, standards and assessment reports from international organizations and various national governments, as well as relevant research literature. Reference to scientific information and research results is an important guarantee for product quality and can enhance the competitiveness of enterprises in the market. To facilitate enterprises to understand the new regulation in a timely manner and make scientific reference, Foodmate here will briefly summarize the adjustments made in the new EU regulation ((EU) 2023/915) on the contaminant limit in formula foods for infant and young children compared with the previous version((EC) No 1881/2006) and summarize the specific requirements for this type of food in the new EU regulation.
1. Overview of Adjustments in the New Regulation
Different countries have different classifications and definitions for infant formula. In China,infant and young children formula is divided into infant formula for babies aged 0-6 months, older infant formula for babies aged 6-12 months, and young children formula for babies aged 12-36 months. In EU, the definition of infant formula is specified in Regulation (EU) No 609/2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control, which includes infant formula and follow-on formula. It specifies that “infant formula” means food intended for use by infants during the first months of life and satisfying by itself the nutritional requirements of such infants until the introduction of appropriate complementary feeding, while “follow-on formula” means food intended for use by infants when appropriate complementary feeding is introduced and which constitutes the principal liquid element in a progressively diversified diet of such infants. There is, however, no definition of young children formula in EU.
For the above-mentioned infant formula and follow-on formula, the major revisions in the new regulation include:
Polycyclic Aromatic Hydrocarbons (PAHs)
The maximum levels for PAHs in infant formula and follow-on formula were set out for commercially available products (The maximum level refers to the product as sold) in previous regulation ((EC) No 1881/2006) without specifying the physical form of the product. This value remains unchanged in the new regulation while specifies that the maximum levels of PAHs is only applicable to products ready to use (placed on the market as such or after reconstitution as instructed by the manufacturer).
Melamine
The previous regulation set a maximum level of 1.0 mg/kg for melamine in powdered infant formula and follow-on formula. The new regulation added 0.15 mg/kg as maximum level for melamine in liquid infant formula and follow-on formula.
![[230612]bc4f50a0b9c661024a96eae12e729cdc.jpeg.jpeg [230612]bc4f50a0b9c661024a96eae12e729cdc.jpeg.jpeg](https://global.foodmate.net/file/upload/image/20230612/[230612]bc4f50a0b9c661024a96eae12e729cdc.jpeg.jpeg)
2. The Limit of Contaminants in Infant Formula and Follow-on Formula in New Regulation
The following table shows the latest EU requirements for contaminant limits in infant formula and follow-on formula.
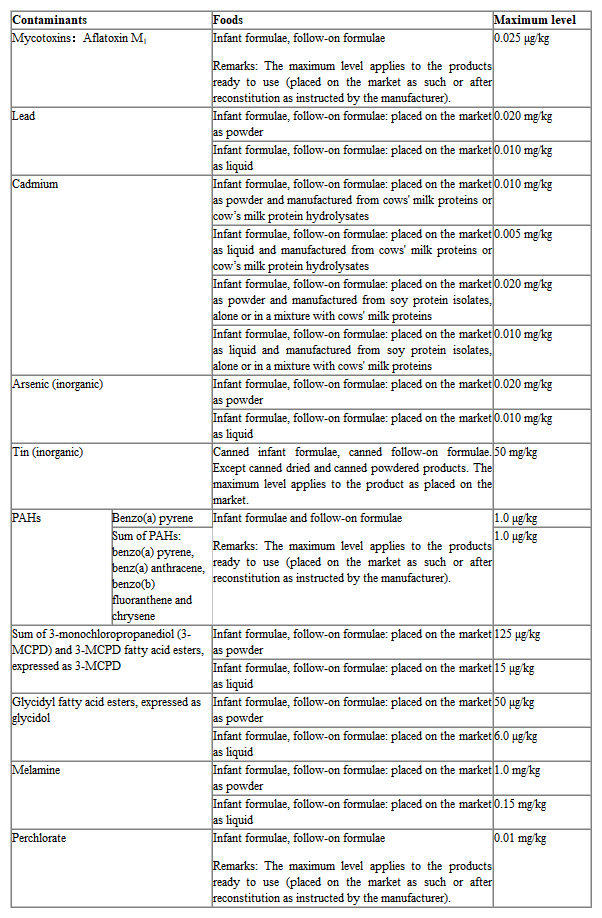
3. Final Words
According to Administrative Measures for the Registration of Product Formula of Infant and Young Children Formula Milk Powder, infant and young children formula produced, sold, and imported within the territory of the People’s Republic of China must have their formula registered and approved by the State Administration for Market Regulation. At the registration stage, manufacturers need to explain the possible hazardous substances in the product, such as contaminants, microorganisms, and mycotoxins, as well as corresponding control plans from the aspects of safety management of raw materials, auxiliary materials, and packaging materials, and safety management of product (e.g., the limit requirements for finished products, regular monitoring system, etc.). This article can provide reference for food enterprises intending to export infant and young children formula milk powder to China on the limit requirements for finished products.
The standard and regulatory system is constantly being revised and supplemented. Foodmate will always keep up with the latest development in standards and regulations. Supported by rich registration experience, Foodmate will provide high-quality registration services for enterprises in need of registration and always welcome inquiries for more information.