
On June 29th, the International Agency for Research on Cancer (IARC), a branch of the World Health Organization, released the latest research findings on aspartame. IARC assessed the potential carcinogenic effects of aspartame, while the Joint FAO/WHO Expert Committee on Food Additives (JECFA) conducted simultaneous risk assessment on aspartame. The evaluation results from both organizations will be published simultaneously on July 14th. According to Reuters, citing insider sources, aspartame is expected to be declared a possible carcinogen. This announcement has immediately sparked widespread attention and discussions among relevant associations and industries.
What Is Aspartame?
Aspartame, also known by its chemical name aspartyl-phenylalanine-1-methyl ester, is a white crystalline powder with a strong sweetness. It was first approved as a food ingredient in 1974 and received approval from the United States Food and Drug Administration (FDA) in 1981.
Aspartame is an artificial non-sugar sweetener commonly used as a sugar substitute in food and beverages. It will lose its sweetness when exposed to temperatures above 80℃, so it is not suitable for cooking or baking. It is typically used in room temperature or cold drinks. In the human gastrointestinal tract, aspartame can be broken down into aspartic acid, phenylalanine dipeptide, and methanol. Therefore, aspartame cannot be detected in the bloodstream even with high intake (200 milligrams per kilogram of body weight).
Due to phenylketonuria, a genetic disorder affecting phenylalanine metabolism, individuals with this condition face significant health risks if they consume foods containing phenylalanine. Aspartame should be labeled as "Aspartame (contains phenylalanine)" according to the regulations of the “Standards for the Use of Food Additives”(GB 2760).
The Carcinogenicity of Aspartame
This is not the first time aspartame has been linked to cancer. In early 2008, Coca-Cola introduced a new sugar-free carbonated beverage called “Zero” in Shanghai. In July, an article titled “Zero: It is Advised to Avoid Drinking!” claimed that the aspartame ingredient in the beverage could cause migraines and even cancer. This article quickly spread on the internet. On July 8th, Coca-Cola China released a statement on its official website refuting the claim, stating, “This accusation is baseless. In fact, over 200 scientific studies have shown no association between the consumption of aspartame and cancer.”
The safety of aspartame has been extensively studied, and it is one of the most rigorously tested food ingredients. JECFA evaluated its safety in 1980 and 1993, establishing an acceptable daily intake range of 0-40 mg/kg. In response to ongoing safety concerns, the European Food Safety Authority (EFSA) conducted safety assessments of aspartame in 1984, 1988, and 2002, endorsing its safety under conditions of use and the daily intake range set by JECFA.
Regarding carcinogenicity, although there have been reports of aspartame inducing tumors in animal studies, official evaluations by JECFA, the U.S. FDA, and the EFSA conclude that aspartame has no carcinogenic effects on animals. In 2005 and 2006, the European Ramazzini Foundation for Environmental Sciences reported research results suggesting that aspartame could cause tumors in multiple organs of animals. However, both the FDA and the EFSA reviewed and assessed the results and original data of these experiments, and did not endorse the interpretation of this research, concluding that aspartame does not have carcinogenic effects.

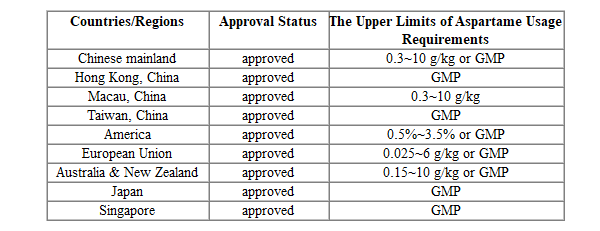
Approval Status of Aspartame Use
Currently, the safety of aspartame has been recognized and approved by most countries, allowing its use as a sweetener in food. The specific usage limits vary depending on the category of food. FoodMate has summarized the upper limits of aspartame usage requirements set by mainstream countries/regions for different food categories.
Table 1 The Upper Limits of Aspartame Usage Requirements Set by Mainstream Countries/Regions For Different Food Categories
Recent Developments in Aspartame Research
Recently, the United States, Canada, and the European Union have provided updates or overviews of their respective approval processes and assessments of aspartame.
The United States has stated that it will continue to monitor the latest scientific research on sweeteners in various ways, closely following published literature and current levels of consumer exposure. They also participate in international scientific activities and standards development related to food safety.
Canada has allowed aspartame as a food additive in various food products since 1981. Health Canada’s Food Directorate has established an acceptable daily intake (ADI) of 40 mg/kg body weight per day, which is the same as the ADI set by JECFA.
EFSA is currently reevaluating the safety of two related food additives: aspartame-acesulfame salt (E962) and neotame (E961). As part of the reevaluation of aspartame-acesulfame salt (E962), EFSA will also update the dietary exposure assessment for aspartame.
Summary
According to the International Council of Beverages Associations (ICBA), IARC does not claim to be an authoritative body for risk assessment based on actual consumption patterns and does not provide health advice. As for whether aspartame truly causes cancer or poses risks to human health with dietary intake, it is advisable to wait for the release of the JECFA safety assessment results in July. FoodMate will continue to monitor and provide relevant interpretations.