
Human milk oligosaccharides (HMOs) are the third largest solid component in human milk, after lactose and lipids. lacto-N-fucopentaose I (LNFP-I) and 2′-fucosyllactose (2'-FL) are both fucosylated neutral oligosaccharides. More than 200 different HMO structures have been detected in human milk, and LNFP-I and 2'-FL are among the five most abundant HMOs.
On December 1, 2023, EFSA announced that the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) had issued an opinion on the use of the lacto-N-fucopentaose I and 2'-fucosyllactose (LNFP-I/2'-FL) mixture as a novel food under (EU) 2015/2283. After evaluation, The Panel concludes that the NF, a mixture of LNFP-I and 2’-FL, is safe under the proposed conditions of use.
On 1 March 2021, the company Glycom A/S submitted a request to the Commission to place the lacto-N-fucopentaose I/2′-fucosyllactose (LNFP-I/2’-FL) mixture on the EU market as a novel food (NF).
2’-FL is included in the Union list of authorised NFs, when chemically synthesised or produced by fermentation by genetically modified strains of Escherichia coli K-12 DH1, E. coli BL21 (DE3) and etc. Moreover, a 2’-FL/difucosyllactose (DFL) mixture produced by a genetically modified strain of E. coli K-12 DH1, and 3-fucosyllactose (3-FL), a constitutional isomer of 2’-FL produced by genetically modified strains of E. coli K-12 MG1655 or E. coli BL21 (DE3) , are also included in the Union list of authorised NFs.
LNFP-I is a fucosylated derivative of lacto-N-tetraose (LNT), which is authorised as a NF when produced by genetically modified strains of E. coli K-12 DH1 or E. coli BL21 (DE3).
The NF submitted in this appication is a powdered mixture mainly composed of LNFP-I and 2’-FL. It is produced by fermentation by a genetically modified strain of E. coli K-12 DH1. The applicant intends to add the NF in a variety of foods, including infant formula (IF) and follow-on formula, foods for infants and toddlers, foods for special medical purposes and food supplements (FS). The target population is the general population. The following figure shows the recommended maximum usage limits for the expected LNFP-I/2 '- FL mixture in various types of food.
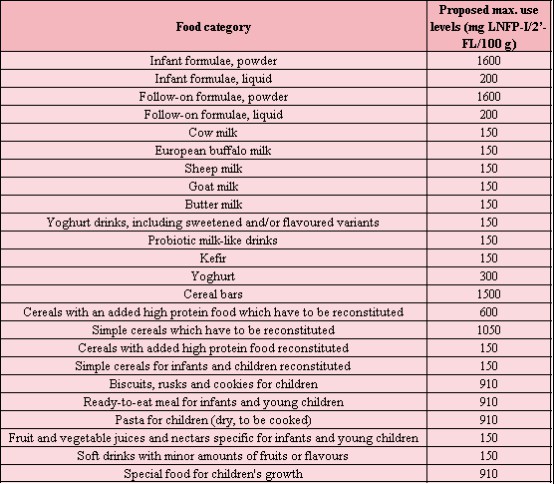
The safety assessment of the components of this NF is mainly based on the comparison between the natural intake of breastfed infants and the estimated intake of NF components. Taking into account the intrinsic nature of HMOs with their limited absorption, the absence of toxicologically relevant effects in the subchronic study and considering that breastfed infants are naturally exposed to these substances, the Panel considers that the consumption of a mixture of LNFP-I and 2’-FL in the NF under the proposed conditions of use does not raise safety concerns.
based on the safety assessment results of the EU on LNFP-I/2 '- FL mixture as a new novel of food, it is likely that LNFP-I/2' - FL mixture will be included in the Union list of authorised NFs in the future. Foodmate will continue to follow relevant progress and share the latest news with everyone in a t imely manner.