
The "Prescribing Management of Functional Foods" issued by the Ministry of Health of Vietnam (hereinafter referred to as the "Amendment") provides detailed regulations on the production, marketing, product announcements, labeling, and usage instructions for functional foods. The Amendment amends and supplements the Circular No. 43/2014/TT-BYT, with the amendments taking effect from November 9, 2023. Foodmate analyzes the main contents as follows.
I. Scope of Application:
The Amendment applies to functional foods, including Supplemented food, Health Supplement, and Medical Food, as well as Food for Special Dietary Uses. It does not apply to infant formula foods, which should follow corresponding technical standards and regulations.
II. Terminology:
"Supplemented food" is retained and defined: "supplemented food" means a normal food that is supplemented with micronutrients and other ingredients food for health such as vitamins, minerals, amino acids, fatty acids, enzymes, probiotics, prebiotics and other biologically active substances .
The definitions of "Health Supplement/Food Supplement/Dietary Supplement", "Food for Special Medical Purposes, Medical Food", and "Food for Special Dietary Uses" have been deleted. The definitions of these products have been introduced in detail in 15/2018/ND-CP.
III. Product Declaration and Registration:
The process for product self-declaration and registration of product conformity requirements have been revised, following the specific regulations of the Vietnamese government regarding food safety. Product self-declaration and registration requirements are detailed in Table 1.
Table 1 Requirements for product self-declaration and registration before and after the amendment
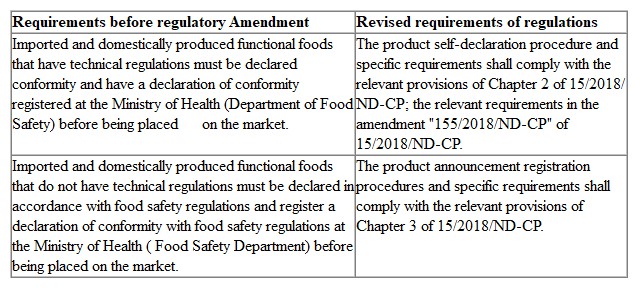
According to the requirements of the amendment order, supplemented food products will need to do the self-declaration in the future; while the remaining three types of functional foods will need to be announced registration.
IV. Labeling Requirements:
Delete some labeling requirements for various functional foods
(1) Supplementary foods:
For Supplementary Foods, there is no need to be labeled as “supplementary food” or the name in the standard specification; nor do they need to specify the recommended dosage or applicable population;
(2) Health supplement
For Health supplement products, the labeling requirement "list of main ingredients that constitute the product's purpose in descending order of volume" has been deleted; such products will no longer have to be labeled "dietary food" to distinguish traditional foods and drugs. When the main ingredient constituting the use of the product is used as the name of the product, “the requirement that the proportion of the active ingredient must be labeled” has been deleted. In addition, the requirements for the height, colour and background design of the labeling of the phrase "This product is not a drug and cannot be used as a substitute for drugs" have been stipulated.
(3) Medical Food and Food for Special Dietary Uses:
For Medical Food and Food for Special Dietary Uses, all labeling requirements have been deleted, including specific labeling requirements for phrases that are different from traditional foods or ordinary foods, relevant usage requirements, warning instructions and other information.
V. Summary:
The Amendment focuses on the consistency between this regulation and the basic food safety regulations, deletes the special labeling requirements for functional foods, and in terms of product self-declaration and announcement registration, uniformly implements the requirements of the basic food safety regulations, and further clarifies the basic attributes of functional foods as foods.
With successful practices and rich experience in Vietnam imported food products self-declaration and announcement registration, Foodmate can guide enterprises to carry out all kinds of food products self-declaration and announcement registration, welcome industry friends to seek further discussion and consultation via global_info@foodmate.net.