
On March 12, China updated and released the "National Food Safety Standard for the Use of Food Additives" (GB 2760-2024), which will replace the "National Food Safety Standard for the Use of Food Additives" (GB 2760-2014) on February 8, 2025. The update of the standard involves changes in the use of food additives in some food categories, mainly including the use of preservatives in canned food, the requirements for the total amount of sweeteners used in the same food category, the cancellation of the use of dehydroacetic acid and its sodium salt in some foods, and the cancellation of the use of hydrogen peroxide, a processing aid in some food processing.
These preservatives will not be allowed in canned foods
The new version of GB 2760 deletes the provisions on the use of food additives for canned food with the sole function of preservatives and the first function of preservatives. These include:
(1) ε-polylysine hydrochloride is no longer allowed to be used in canned fruits, canned vegetables, nuts and seeds;
(2) Lactic acid streptococcin and ε-polylysine hydrochloride are no longer allowed to be used in edible fungi, canned algae and canned grains;
(3) ε-polylysine, ε-polylysine hydrochloride, lactic acid streptococcin, sodium diacetate (also known as sodium diacetate), sorbic acid and its potassium salt, dehydroacetic acid and its sodium salt (also known as dehydroacetic acid and its sodium salt) are no longer allowed to be used in canned meat;
(4) Steady chlorine dioxide is no longer allowed to be used in canned aquatic products.
A new requirement for the total amount of sweeteners to be used together
The new version of GB 2760 has added the total amount requirements for aspartame, acesulfame potassium and aspartylphenylalanine methyl ester acesulfame when used together in the same food category. The specific provisions are as follows:
(1) Aspartame (also known as aspartylphenylalanine methyl ester): If aspartame-acesulfame salt is allowed to be used in the food category at the same time (the maximum dosage multiplied by 0.64 can be converted to the dosage of aspartame), when mixed, the maximum dosage cannot exceed the maximum dosage of aspartame specified in the standard.
(2) Aspartame-acesulfame salt: If aspartame or acesulfame potassium is allowed to be used at the same time in the food category, the maximum dosage of aspartame or acesulfame potassium specified in the standard shall not exceed the maximum dosage of aspartame or acesulfame potassium specified in the standard (the maximum dosage of aspartame-acesulfame salt multiplied by 0.64 can be converted to the dosage of aspartame, and the maximum dosage multiplied by 0.44 can be converted to the dosage of acesulfame potassium).
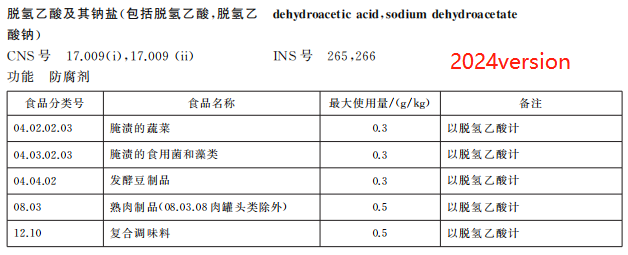
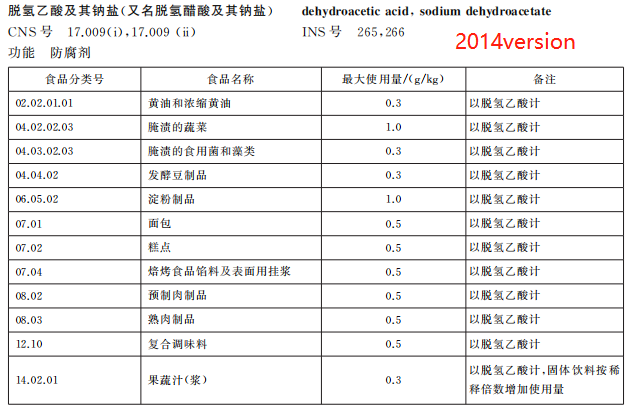
Remove the use of dehydroacetic acid and its sodium salts in 7 types of foods such as pastries
According to the food safety risk assessment results of the food additive sodium dehydroacetate organized by the Food Assessment Center, combined with the investigation of the use of sodium dehydroacetate in related industries, the new version of GB 2760 deletes the provisions on the use of dehydroacetic acid and its sodium salt in butter and concentrated butter, starch products, bread, pastries, baked food fillings and surface hanging pulp, prefabricated meat products, fruit and vegetable juice (pulp), and adjusts the maximum use of the food additive in pickled vegetables, from 1.0g/kg to 0.3g/kg.
Limit the use of hydrogen peroxide, a processing aid
based on the latest assessment results of safety and process necessity, combined with the actual use of the industry, the specific function and scope of hydrogen peroxide when used as a processing aid were clarified, and the processing aid hydrogen peroxide was adjusted from the "List of Processing Aids that Can Be Used in the Processing Process of Various Foods with No Limit on Residues" to the "List of Processing Aids that Need to Specify the Functions and Scope of Use", and stipulated that the processing aid hydrogen peroxide can be used as a desulfurizer, decolorizing agent and deiodizer, which can be used for starch sugar and starch processing technology, oil processing technology, seaweed processing technology, collagen casing processing technology, whey powder and whey protein powder processing technology.
Delete some of the regulations on the use of food additives that are not necessary for the process
According to the investigation of the beverage industry, in accordance with the principle that the use of food additives should be necessary for the process, the provisions on the use of natamycin in fruit and vegetable juice pulp were deleted.
With the improvement of the production process, taking into account the principle of process necessity, the regulations on the use of β-carotene and diacetyl tartrate in distilled spirits were deleted, and the regulations on the use of azoformamide in wheat flour were deleted.
based on the principle of process necessity, the provisions for the use of the processing aid β-cyclodextrin for pasteurized milk and sterilized milk have been deleted.
Final Suggestion
Foodmate reminds overseas enterprises exporting food to China to review the formula in a timely manner to ensure that the products continue to meet the requirements of Chinese regulations. In addition, we can provide professional food import and export consulting services to help you avoid the risk of non-compliance of food additives, if you need, please contact us via global_info@foodmate.net.