
On July 18, 2024, the Ministry of Health of Vietnam issued the national technical regulations for the limits of contaminants in health supplement products, QCVN 20-1: 2024/BYT, through the issuance of Circular No. 12/2024/TT-BYT, which will come into effect on August 1, 2025. The circular also clarifies the transitional measures for registered health supplement products: For health supplement products that have been produced before the effective date of this circular and have obtained product announcements and registration, if they do not comply with the standards stipulated in the annex of this circular, organizations and individuals may continue to import, operate, and circulate until the end of the product's shelf life, unless the product is reported due to food safety issues.
I. Product Definition and Scope of Application Standards
According to the provisions of Article 3, Paragraph 1 of the Government Decree No. 15/2018/ND-CP: Health supplement products refer to products used to supplement daily diet to maintain, enhance, or improve human body functions, and reduce the risk of diseases. Health supplement products contain one or more of the following ingredients:
a) Vitamins, minerals, amino acids, fatty acids, enzymes, probiotics, and other substances with biological activity;
b) Ingredients from natural sources, including extracts, isolates, concentrates, and derivatives from animals, plants, and botanicals;
c) Synthetic sources of the ingredients mentioned in points a) and b).
This technical standard specifies the maximum limits for contaminants (heavy metals and microorganisms); sampling and testing methods; management requirements; and the responsibilities of organizations and individuals producing and trading health supplement products. This standard is not applicable to tonic wine products that are announced as health supplement products.
II. Requirements for heavy metal limit in health food products
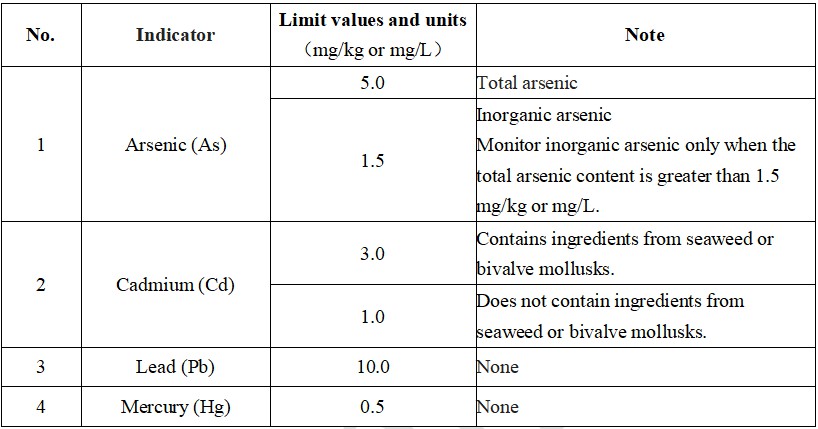
III. Microbial index requirements for health supplement products
In terms of microbial limits, the standard categorizes Health supplement products into the following six types based on their ingredient composition:
(1) Health supplement products containing plant components, which must be treated with boiling water according to instructions before use (boiling water soaking, blanching, etc.); for example: herbal teas.
(2) Health supplement products containing plant components.
(3) Health supplement products that are mixtures containing ingredients from animals, minerals, or both animals, minerals, and plants.
(4) Health supplement products containing one or more components: vitamins, minerals, amino acids, fatty acids, enzymes, biologically active substances with identified chemical activity, and not belonging to the aforementioned groups (1), (2), and (3).
Water-containing forms (where water is a part of the product formula) (for example: aqueous solutions, syrups, mixtures, concentrated liquids, jellies...).
(5) Health supplement products containing one or more components: vitamins, minerals, amino acids, fatty acids, enzymes, biologically active substances with identified chemical activity, and not belonging to the aforementioned groups (1), (2), and (3).
Non-water-containing forms (where water is not a part of the product formula) (for example: compressed tablets (regular compressed tablets, crushed compressed tablets, film-coated tablets), soft capsules, hard capsules, granular, powdered, film, gummies, oil-based...).
(6) Health supplement products that only contain probiotics.
The microbial indicators for health supplement products with different formula compositions mainly involve: total aerobic microbial count, total yeast and mold count, Escherichia coli, Salmonella spp. and Staphylococcus aureus. Taking group (6) products as an example, the microbial indicator requirements are as follows:
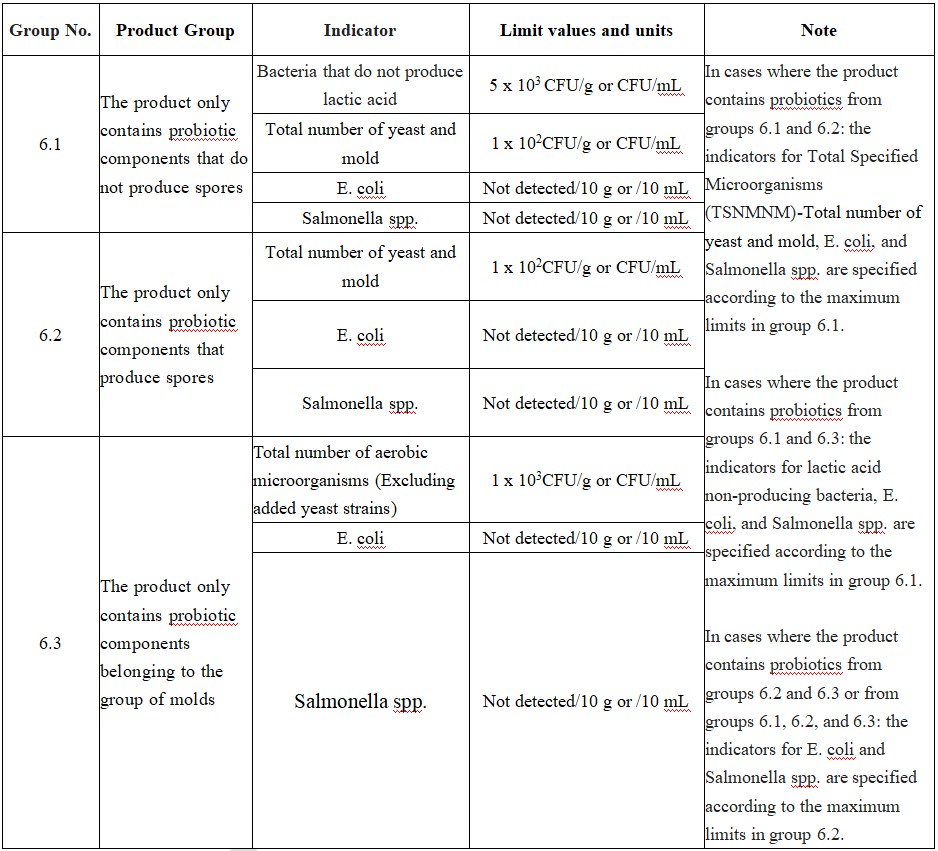
Previously, Vietnam had not established specific limits for microbial and heavy metal content in health food products. The release of this Circular marks a further improvement in Vietnam's health food safety system. Companies interested in exporting health food products to Vietnam should ensure that the levels of microorganisms and heavy metals in their products meet the standard requirements before exporting. It is essential to test and confirm these indicators during product registration. For more detailed requirements, please contact Foodmate via global_info@foodmate.net for consultation and acquisition.