
On October 18, 2024, the website of the French National Agency for Food, Environment and Occupational Health Safety (Anses) announced that Anses, at the request of the Directorate General of Food (DGAL), has given expert opinion on the updating of the decree of May 9, 2006 on the nutrients available in dietary supplements, which includes the following main points:
(1) Labeling Warnings: The draft decree provides for labeling contents for specific groups of people, and the Committee of Experts on Human Nutrition (CES) evaluated and recommended adjustments to some of the labeling contents, for example, it was considered that Vitamin A is not relevant for labeling for pregnant women and menopausal women, and that Beta-carotene should be labeled with warnings for smokers;
(2) New labeling recommendations: the recommendation to label Vitamin E as "not recommended for use by persons undergoing anticoagulation therapy"; a reminder of the risks associated with excessive intake of potassium; and special requirements for the labeling of Vitamin B9 in supplements for pregnant women;
(3) For infants and young children: the regulation requires reinforcement: support for strengthening the requirements for medical supervision of vitamin and mineral supplementation for infants (0-1 year olds) and young children (1-3 years olds), and clarification that restrictions should be placed on the label. Supplements are not recommended for infants without complementary feeding unless medically necessary; age limits are clarified: it is considered that the category "children (<10 years)" should exclude young children aged 0-3 years;
(4) Revision of the maximum daily dose (DJM) of some nutrients, as shown in the table below:
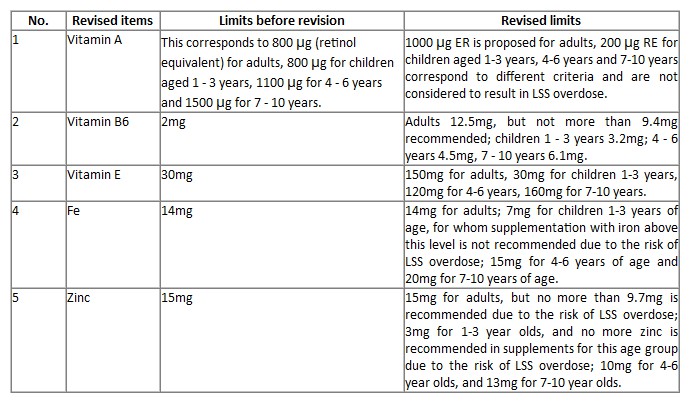
Learn more: https://www.anses.fr/fr/system/files/NUT2023SA0165.pdf