Recently, Department of Agriculture, Water and the Environment (the Department) issued an imported Food Notice - IFN 02-21 - Changes to tests applied to surveillance food to advise importers and brokers of changes to tests applied to surveillance food that come into effect for all entries lodged from 19 July 2021. Foodmate will share relevant key information with you in response to this imported Food Notice.
1 Background
imported food is inspected by the Department through an inspection program known as the imported Food Inspection Scheme (IFIS). Under the IFIS, the Minister classifies food as either risk food or surveillance food. Risk food is food that has been assessed by Food Standards Australia New Zealand (FSANZ) as posing a medium to high risk to public health, thereby requiring stricter border controls. Surveillance food is considered to pose a low risk to human health and safety. There are many standards in the Australia and New Zealand Food Standards Code (the Code) and it is not practicable to inspect against all standards, particularly for low-risk food. The department inspects imported food against a selection of standards but not all standards. Tests that apply may change from year to year to ensure compliance against different standards over time. Changes to existing tests or the introduction of new tests may also occur if the department receives new or updated risk advice from FSANZ.
2 Revisions
Schedule 27 of the Code has two microbiological limits, Salmonella and Cronobacter that apply to infant formula and one microbiological limit, Salmonella, that applies to infant follow-on formula. The limits in the Code reflect those set by the Codex Alimentarius Commission (Codex), the international food standards setting body. Introducing Cronobacter testing for infant formula and applying Salmonella testing to follow-on formula will allow them to verify that imported product is compliant with the limits in the Code for these pathogens.
Besides, imported finfish have been targeted for testing for antimicrobial residues based on a list of fish species known to be raised under aquaculture conditions since August 2017. The department is aware that there are significant quantities of milkfish (Chanos chanos) of the Chanidae family being imported into Australia. Milkfish is typically raised under aquaculture conditions and are potentially exposed to antibiotics during their production. As milkfish was not previously considered in scope for the antimicrobial residue screen, it is unknown if this food is compliant with the Code regarding chemical (antimicrobial) residues. The list of fish species targeted for the antimicrobial residue screen will be increased to include Chanos chanos (milkfish) of the Chanidae family.
The following table is a summary of the upcoming changes in food categories and their testing requirements.
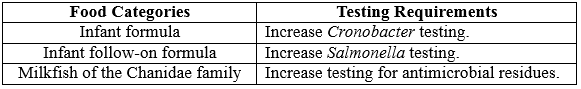
3 Summary
Australia will revise the inspection requirements for some surveillance foods, which is expected to have a greater impact on the export trade of dairy powder and fish from various countries, so please keep an eye on the new regulations and be prepared in advance to avoid trade losses. In addition, Foodmate will also pay attention to the subsequent revision of regulatory developments, to give export enterprises timely reminders and analysis.