On, June 30th, 2021. the official account ‘12360 customs hotline’ has issued General Scientific Articles about certificate of imported food. Foodmate has translate this article into English to help overseas enterprises easily understand the process of imported food by three articles.
Let’s look at the first part- Registration certificate of formulated food for special medical purposes
From January 1st, 2021, the General Administration of Customs further combed the current supervision certificates in import and export links, merged two kinds and cancelled one on the basis of reducing the original 86 kinds of supervision certificates in import and export links to 44 kinds. So far, the supervision certificates in import and export links have been reduced to 41 kinds. Among which, the supervision certificates related to food import include registration certificate of formulated food for special medical purposes, registration certificate of health food or record certificate of health food, certificate of formula registration for infant formula milk powder, and certificates issued by some foreign official agencies of imported food, such as certificate of origin, the certificate of radioactivity test and the certificate of origin issued by the official organization of Japan.
Currently, Enterprises should apply to the State Administration for Market Regulation and its authorized institutions for registration certificate of formulated food for special medical purposes, registration certificate of health food or record certificate of health food, certificate of formula registration for infant formula milk powder.
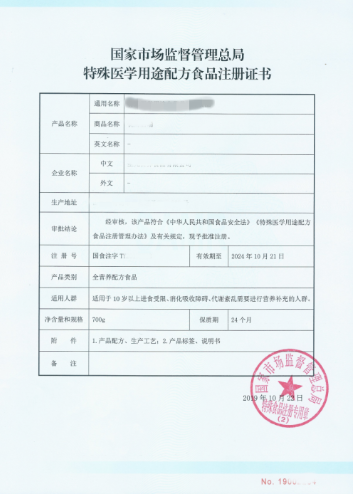
Registration certificate of formulated food for special medical purposes
Formula foods for special medical purposes:as especially formulated foods that are produced to meet the special requirements for nutrient or meals of people who suffer from eating limitation, disorder of digestion and absorption, metabolic disorders or special disease state, these products shall be eaten individually or with other foods under the guidance of doctors or clinical dietitians.
(GB 29922-2013 National Food Safety Standard General Rule on Formulated Foods for Special Medical Purposes)

Legislative authority: Food Safety Act of People’s Republic of China, Administrative measures for registration of formulated food for special medical purposes.
Application conditions: The applicant for registration of formulated food for special medical purposes shall be the manufacturer who intends to produce and sell formulated food for special medical purposes in China and the overseas manufacturer who intends to export formulated food for special medical purposes to China.
Application documents:
- 1. Application form for registration of formulated food for special medical purposes;
- 2. Product R&D report and product formula design and their basis;
- 3. Production process materials;
- 4. Product standard requirements;
- 5. Sample of product label and instruction manual;
- 6. Inspection report of test sample ;
- 7. Certification materials for proving capability of R & D, production and inspection ;
- 8. The clinical trial report shall also be submitted when applying for nutritionally complete food with a nutrient-adapted formulation specific for a medical condition;
- 9. Supporting documents related to registration application.
Handling procedures:

Name and sample: Registration certificate of formulated food for special medical purposes, valid for 5 years.
Source and download website of information:
http://www.samr.gov.cn/fw/wsbs/sp/tsspzc/
(Source: State Administration for Market Regulation)
The above are detailed information for how to obtain Registration certificate of formulated food for special medical purposes, please look forwards for next part-Registration certificate of health food or record certificate of health food.