Core Tip: On March, 2020, National Medical Products Administration (NMPA) announced 5 batches of information on approved health foods, respectively on March 3, 5, 6 and 17, and 2 batches of unapproved health food published on March 9 and 17 (69 rejected applications). Since March 25, 2020, NMPA did not reveal the case number and the registration situation (approved or not) any more, shown in information released on March 25 and 27.
Global Foodmate summarised the published information of approved health food in March, 2020, for your reference.
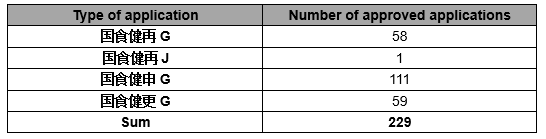
In March, 2020, 229 health foods have been approved, namely 58 re-registration (Domestic) (国食健再 G) and 1 re-registration (imported) (国食健再 J), 111 newly registration (国食健申 G), 59 alteration (国食健更 G), as follows:
To understand the health food registration certificate
Extending reading:
Evolution of Health Food Registration and Filing Number Format
The Management of China Health Food Registration Certificate
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net