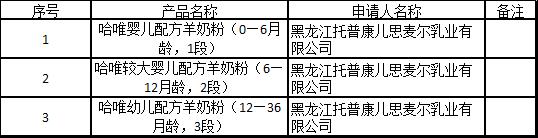
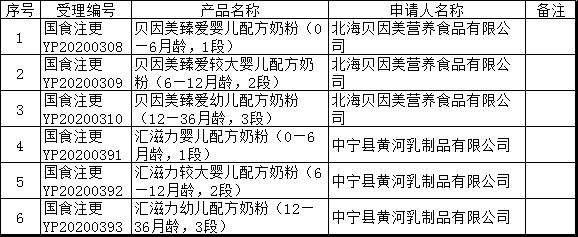
On December 16, the Center of Food e
valuation of State Administration for Market Regulation updated the list of information to be received for approval docu
ments and the notice of e
valuation opinions, in which the names of products involved Beingmate Zhenai infant formula (0-6 mo
nths old, stage one), etc.
12月16日,国家市场监督管理总局食品审评中心更新批件待领取信息及审评意见通知书清单,其中涉及产品名称为贝因美臻爱婴儿配方奶粉(0—6月龄,1段)等。
For more details:
http://www.cfe-samr.org.cn/files_zybh/pub_xxc/tmp/20201207.html
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net