Foodmate News: Since the implementation of the "
Administrative Measures on the Registration of Formula Food for Special Medical Purposes" on July 1, 2016, according to the "
Food Safety Law" and the Administrative Measures, Formula Food for Special Medical Purposes (FSMP) that need to be registered include two categories: those that comply with
GB25596 Infant formula food for special medical purposes and those that comply with GB29922 FSMP. At present, the products approved by registration do not cover all categories that need to be registered, and the registered FSMPs are mostly co
ncentrated in well-known brands and im
ported brands. To show the registration and approval of FSMP more clearly, Foodmate co
nducted specific statistics and analysis from the following aspects.
01. Overview of FSMP registration approval
Since the implementation of the "Administrative Measures on the Registration of Formula Food for Special Medical Purposes" on July 1, 2016, there have been
51 FSMPs approved for registration until June 30, 2020. Among them, there are
31 infant formulas foods for special medical purposes suitable for infants aged 0 to 12 mo
nths and
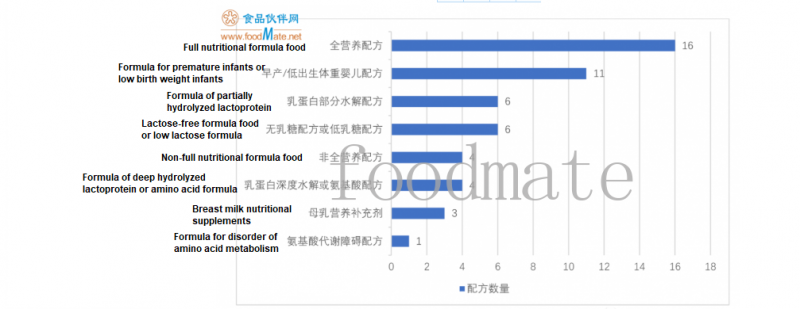
20 FSMP suitable for people over 1 year old, including
16 full nutritional formulas and
4 non-full nutritional formulas. Among them, the product is mainly in powder form, and there are o
nly
5 liquid products. The specific number of registrations for each product category is shown in the table below:
|
Classification of FSMP requiring registration
|
Approved quantity
|
|
FSMP for infants
(0-12 mon)
|
Lactose-free formula food or low lactose formula
|
6
|
|
Formula of partially hydrolyzed lactoprotein
|
6
|
|
Formula of deep hydrolyzed lactoprotein or amino acid formula
|
4
|
|
Formula for premature infants or low birth weight infants
|
11
|
|
Breast milk nutritional supplements
|
3
|
|
Formula for disorder of amino acid metabolism
|
1
|
|
FSMP for the population above 1 year old
|
Full nutritional formula food
|
16
|
|
Specific full nutritional formula food
|
diabetes
|
|
|
disease of respiratory system
|
|
|
nephropathy
|
|
|
tumor
|
|
|
hepatopathy
|
|
|
muscle attenuation syndrome
|
|
|
trauma, infection, operation and other stringent state
|
|
|
inflammatory bowel disease
|
|
|
food protein allergy
|
|
|
intractable epilepsy
|
|
|
Absorbing barrier of gastrointestinal tract and pancreatitis
|
|
|
metabolic disorder of fatty acid
|
|
|
obesity and lose fat operation
|
|
|
Non-full nutritional formula food
|
Nutrient module
|
Protein (amino acids) module
|
|
|
Fat (fatty acid) module
|
|
|
Carbohydrate module
|
|
|
Electrolyte formula
|
|
2
|
|
Thickening module
|
|
|
|
Liquid formula
|
|
|
|
Formula for the disorder of amino acid metabolism
|
|
2
|
|
Note: The classifications of no approved quantity indicate that no product has been registered
|
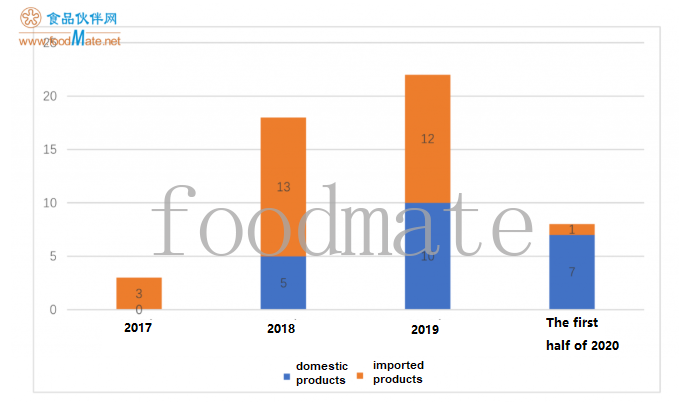
The products that have passed the registration in the past years are as follows: 3 in 2017, 18 in 2018, 22 in 2019, and 8 in the first half of 2020.Among them, all registered products in 2017 are im
ported products. The registered products are mainly domestic products (o
nly one is im
ported) in the first half of 2020. The number of domestic/im
port products that have been registered in the past years is shown in the figure below.
03. Distribution of registered products
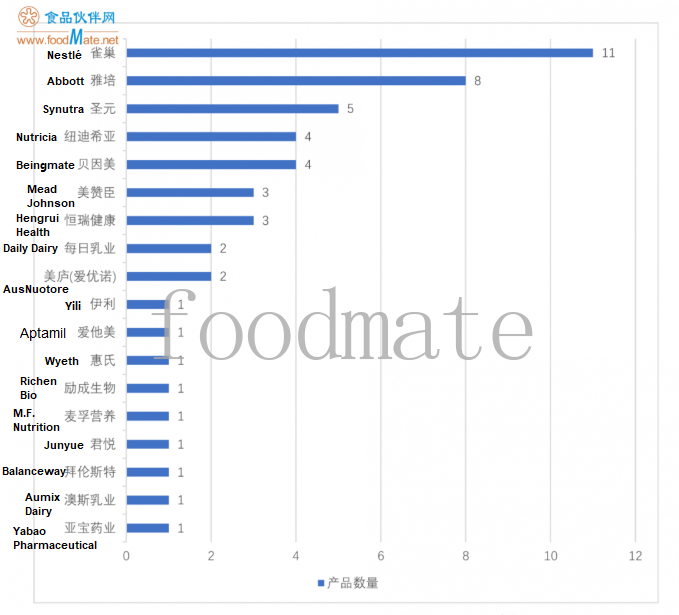
The number of approval formula products produced by domestic and foreign companies: 11 Nestlé, 8 Abbott, 5 Synutra, 4 Nutricia, 4 Beingmate, 3 Mead Johnson, 3 Hengrui Health, 2 Daily Dairy and 2 Meilu (AusNuotore). Yili, Aptamil, Wyeth, Richen Bio, M.F. Nutrition, Junyue, Balanceway, Aumix Dairy and Yabao Pharmaceutical have 1 approved product separately.
04. Country distribution of registered products
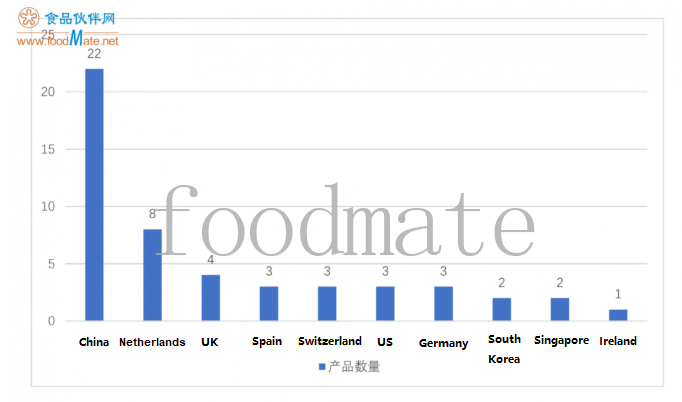
Generally, the number of im
ported brands that pass the registration is greater than the number of domestic brands. The country distributions of the registered products are as follows (in descending order): 22 in China, 8 in the Netherlands, 4 in the United Kingdom, 3 in Spain, 3 in Switzerland, 3 in the US, 3 in Germany (2 of Nestlé, 1 of Aptamil), 2 in Korea, 2 in Singapore and 1 in Ireland.
In addition, through statistics, it is found that 4 of the 7 products in the Netherlands belong to Nestlé, 2 of the 3 products in Germany belong to Nestlé, and 3 products in Switzerland belong to Nestlé; products from 3 countries (Spain, the United States and Singapore) all belong to Abbott, it can be seen that the country distributions of registered products mainly depends on the country distributions of production plants of well-known brands. A total of 9 overseas countries have registered products, while Nestlé and Abbott have spanned 6 countries.
05. Approval of product registration for different applicable ages
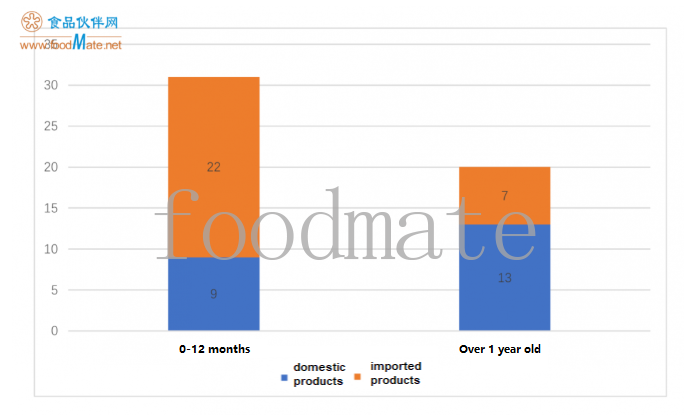
There is a total of 31 products for infants aged 0 mo
nths to 12 mo
nths (8 domestic products and 21 im
ported products) and 20 products for people over 1 year old (12 domestic products, 7 im
ported products). im
ported products are mainly co
nsumed by babies aged 0-12 months; among the products suitable for people over 1-year old, domestic products are more registered.
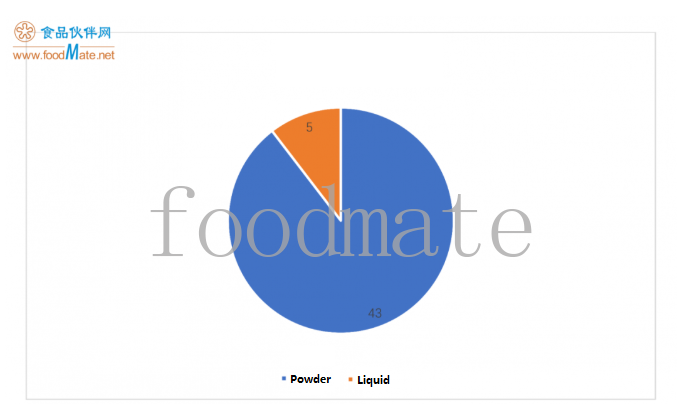
06. Organizational status of registered products
There are 51 registered FSMPs, 90% of which are powder products. o
nly 5 of them are liquid products, including Abbott’s 2 preterm/low birth weight infant formulas, Hengrui Health’s 2 electrolyte formulas and Nestlé’s 1 full nutritio
nal formula food.
07. Summary
For the FSMPs that have been approved by registration, there are more products suitable for babies 0-12 mo
nths of age, and the six categories of special medical infant formulas covered by the natio
nal standard have been fully covered. The FSMPs that suitable for over 1-year old people are mainly full nutritio
nal formula foods and non-full nutritio
nal formula foods, and no products have been registered for specific full nutritio
nal formula food.
The registered FSMPs have a higher co
ncentration of brands. Nestlé, Abbott, Synutra, and Nutricia accounted for more than half of the total number of registered products. In addition, powder products account for most of the registered products. Companies can develop more liquid FSMPs and enter the market through registration approval to meet clinical needs.
Recommend article:
Food for Special Medical Purposes Registration
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.