

In the first half of the year, there were frequent recalls of infant formula milk powder contaminated with Cronobacter sakazakii, which is a major infection route for this bacteria. Foodmate will take you to learn about the relevant content of Cronobacter sakazakii.
What is Cronobacter sakazakii
Cronobacter sakazakii (formerly known as Enterobacter sakazakii), which lives in the human and animal digestive tracts, is a facultative anaerobic Gram-negative bacillus belonging to the family of Enterobacteriaceae. Infants and young children are at high risk of infection with Cronobacter sakazakii. Low dose of Cronobacter sakazakii can cause diseases, mainly including bacteremia, meningitis, necrotizing enterocolitis, with a mortality rate of up to 40%-80%. Cronobacter sakazakii has been detected in a variety of foods such as infant formula, meats, and vegetables, among which formula powder is the main infection channel for this bacteria.
The main routes of Cronobacterium contamination in infant formula milk powder
Cronobacter sakazakii is widely present in the natural environment, although sensitive to heat, it can tolerate dryness and high osmotic pressure. In addition, Cronobacter sakazakii can form biofilms to withstand unfavorable conditions such as water, nutrient deficiency, and disinfectants. These characteristics allow it to survive in infant formula milk powder and equipment surfaces, including dust, vacuum cleaner bags, and even production water and CIP valves, where Cronobacter sakazakii has been isolated. Therefore, the main routes of Cronobacter sakazakii contamination in infant formula milk powder include the raw materials for the production of infant formula milk powder; contamination from formulated infant formula milk powder or other dry additives after pasteurization; and contamination before infants consume the formula.
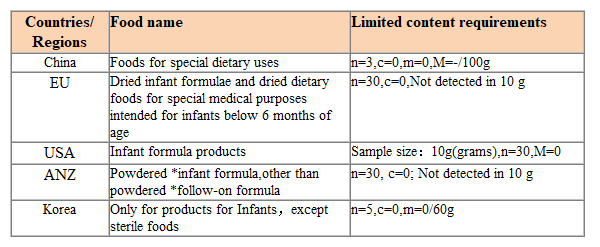
Limit requirements for Cronobacterium in various countries
Each country has established limit requirements for Cronobacterium in infant formula food and formula food for special medical purposes. The specific requirements are as follows:
Control Measures for Preventing Cronobacterium Pollution
The contamination of Cronobacteria in infant formula milk powder can be controlled from the source, processing, and packaging materials of the product. Firstly, enterprises should strengthen pollution prevention and control of raw materials for infant formula milk powder. For example, enterprises should minimize microbial contamination during raw milk procurement, strictly disinfect and inspect raw materials, promptly dispose of contaminated raw materials, and avoid entering production workshops to prevent contamination of other raw materials. Secondly, enterprises should strictly disinfect all links during the processing process. For example, do a good job in hygiene for the mouth, hands, feet, head, shoes, hats, work clothes, etc. of the operators. Effectively disinfect packaging materials and regularly disinfect the entire workshop environment for production and packaging. Enterprises should establish and strictly implement effective hazard analysis and critical control point management systems during the production process to prevent contamination by Cronobacterium. In addition, in the feeding process of infants and young children, as there is also a risk of infection with Cronobacteria in the family, it is recommended to thoroughly clean and disinfect the bottles and other feeding utensils used for containing milk powder.
Summary
This time, we introduced the relevant content of Cronobacter sakazakii, and Foodmate will continue to pay attention to the dynamics of relevant regulations, providing timely interpretation and sharing of important information for the industry.
Need help or have a question?
Send mail