

On September 6, 2019, National Medical Products Administration (NMPA) announced the information on the registration of infant formula (unapproved), involving 3 formulas from 1 company.
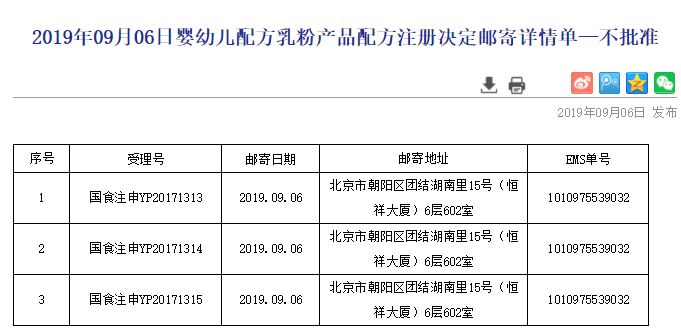
The published case numbers of 3 formulas were 国食注申YP20171313, 国食注申YP20171314 and 国食注申YP20171315. The applicant was not revealed, only the address was published as a building in Tuanjiehu, Chaoyang District, Beijing, China.
For more information in Chinese, please click.
Please note: Original English article of Global Foodmate of Information Service and Business Department, please indicate the source from the Global Foodmate if reprint.
Global Foodmate Information Service Centre provides food standards & regulations research, labeling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net
Need help or have a question?
Send mail