Core Tip: On
December, 2019, Natio
nal Medical Products Administration (NMPA) announced
7 batches of the information on the registration of Infant Formula
(approved), respectively on
December 2, 4, 5, 6, 10, 19 and 26). And 1 batch of unapproved infant formula published on December 25 (9 rejected registration from 1 company).
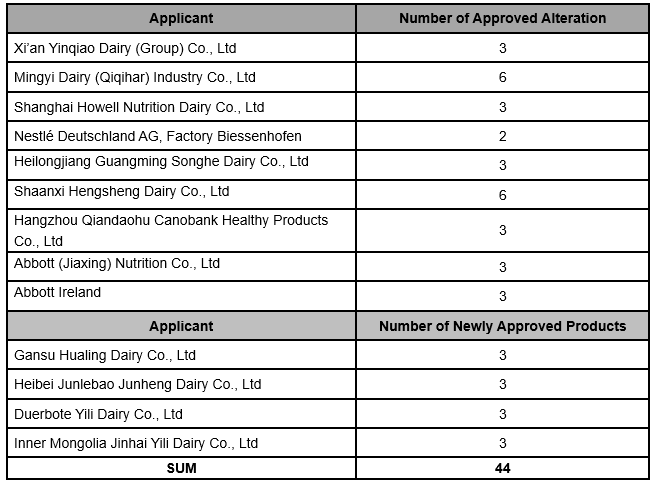
Global Foodmate summarised the published information of approved Infant Formula in December, 2019,
involving 32 formulation alteration from 9 companies and 12 newly approved formulation from 4 companies.
The situation of approved infant formula in December, 2019 is as follows:
Please note: Original English article of Global Foodmate of Information Service and Business Department, please indicate the source from the Global Foodmate if reprint.
Global Foodmate Information Service Centre provides food standards & regulations research, labeling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net