Since the implementation of the health food registration and filing system, until June 30, 2020, the Food e
valuation Center of the State Administration for Market Regulation has published a total of 74 im
ported health food information for filing. The approval status is as follows:
General introduction
Notes:
A. In 2018, 40 record information was released, of which 3 product companies applied for cancellation of record.
B. This statistics is divided into years ba
sed on the approval time of the product filing certificate, and the deadline is June 30, 2020 (data from the official website of the Food e
valuation Center of the State Administration for Market Regulation).
1. Status of related enterprises of imported health food
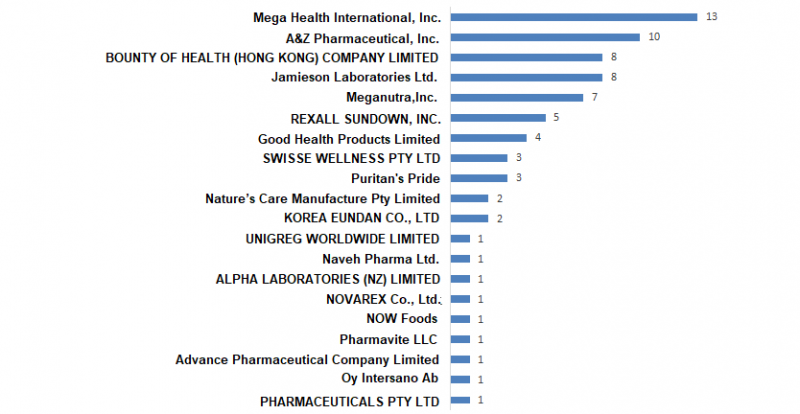
Until 30 June 2020, a total of 20 health food companies have obtained im
port health food registration certificates. The product registration status of these 20 companies is shown in Figure 1. Among them, Mega Health Internatio
nal Co., Ltd. has obtained 13 health food registration certificates, and the number of products ranked first.
Figure 1. Product quantity statistics of health food companies
2. The country or region where the registered health food is imported from
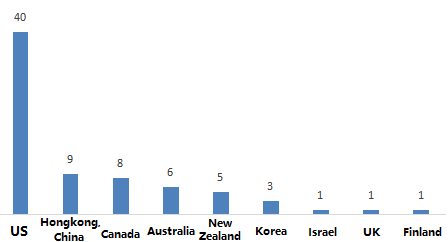
2.1 According to the statistics of the country or region wher
e the filed company is located, the 74 im
ported products currently filed are from 9 countries or regions. Among them, the United States ranked first with 40 products, which accounted for 54.05% of im
ported registered products.
Figure 2. Statistics on the number of countries or regions where health food filing companies are located
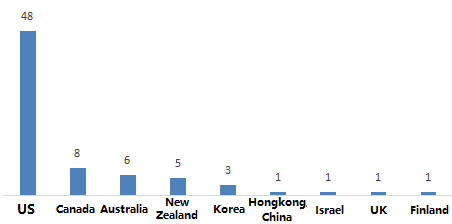
2.2 According to the statistics of the country or region of the production company, the 74 im
ported products currently registered are produced and processed in 9 countries or regions. Among them, the United States ranked first with 48 products, Canada ranked second with 8 products, and Australia ranked third.
Figure 3. The number of health foods manufacturers’ country or region
Analysis:
From the country or region distribution of the above filing companies and production companies, we can see:
A. The location of the recorder of some products is inco
nsistent with the location of the production company, which makes the analysis of the geographical distribution of products from different dimensions have different results.
B. The United States accounts for a large proportion of both the number of filing applicants and the number of product manufacturers.
3. Dosage forms of imported health food products
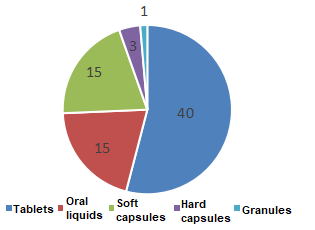
At present, the dosage forms of im
ported health food products for record include tablets, soft capsules, hard capsules, granules, and oral liquids (including dro
ps) (see Figure 4 for details). Among the currently valid filling 74 im
ported products, there are 40 tablets, which are closely related to the nature of the raw materials and the characteristics of the product formulation.
Figure 4. Number of health food products in different dosage forms
Analysis:
A. Through statistics, we found that there is o
nly one product of im
ported registered health food granules, which may because the most foreign products direct fill of into powder after mixing.
B. The tablets of the registered products include normal swallow tablets, lozenges, chewable tablets, and effervescent tablets. At present, normal swallow tablets have a relatively large proportion of the tablet products, with 31 products, followed by 9 chewable tablets. There are no products with lozenges and effervescent tablets to obtain the record certificate.
C. The oral liquid dosage forms of the registered products include drops and oral liquids. Among the 15 oral liquid products that have obtained registration certificates, drops account for a relatively large proportion of 11.
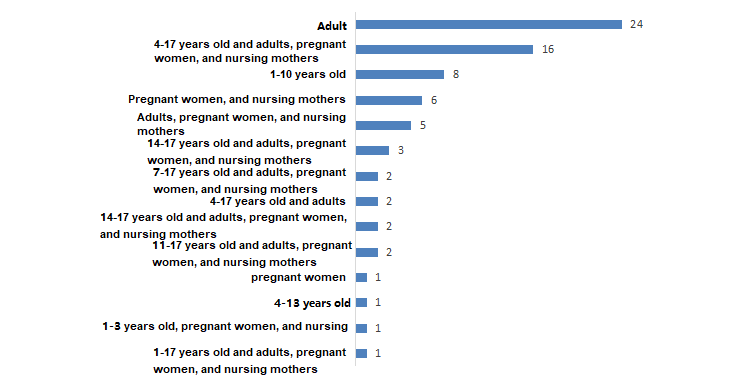
4. Statistics of product suitable population
At present, the suitable population of valid filling im
ported health foods covers all populations of 1-17 years old, pregnant women, nursing mothers and adults. There are 14 kinds of population combinations. Among them, the number of products suitable for "adults" is 24, accounting for 32.43% of the im
ported products, which is the highest proportion. 16 products suitable for "4-17 years old and adults, pregnant women, and nursing mothers", ranking second.
Figure 5. Statistics of suitable populations of registered health food
Analysis:
Overall, im
ported registered health foods are more suitable for special populations such as children, pregnant women, and nursing mothers.
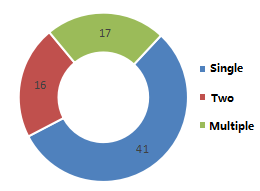
5. Number of supplementary nutrients
According to the number of nutrient supplement products, the registered products can be divided into single nutrient supplement products, two nutrient supplement products, and multiple (three or more) nutrient supplement products. 41 single nutrient supplement products account for 55.41% of all im
ported registered products.
Figure 6. Statistics of supplementary nutrient types of registered health foods
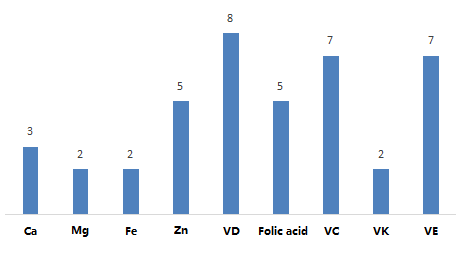
5.1 Nutrient selection of single nutrient supplement products
41 single nutrient supplement products involve supplementation of 9 nutrients, including 4 minerals of calcium, magnesium, iron, zinc, and 5 vitamins of vitamin D, folic acid, vitamin C, vitamin K, vitamin E.
Figure 7. Statistics on single nutrient supplement products in registered health foods
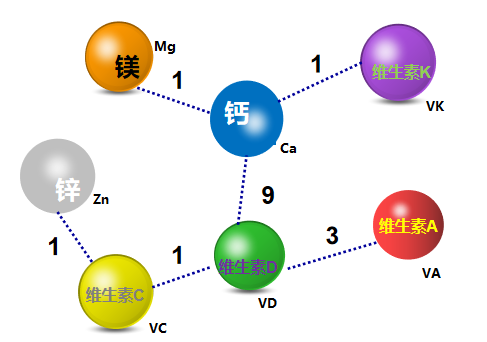
5.2 Nutrient selection of two nutrient supplement products
There are 16 two nutrient supplement products. It mainly involves 6 types of combinations: calcium + vitamin D (9), vitamin A + vitamin D (3), vitamin C + vitamin D (1), calcium + magnesium (1), vitamin C + zinc (1), Calcium + Vitamin K (1).
Figure 8. Statistics on two nutrient supplement products in registered health foods
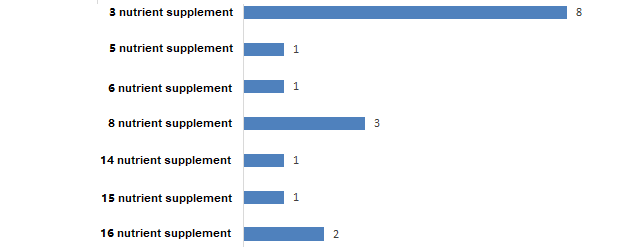
5.3 Nutrient selection of multiple nutrient supplement products.
A total of 17 products supplemented with multiple nutrients (see Figure 9 for details). Among them, the maximum number of supplementary nutrients reaches 16, and there are 2 such products. The types of nutrients supplemented by these two products are the same, which are within the range of 22 nutrients that are currently allowed to be supplemented, except for calcium, magnesium, potassium, copper, pantothenic acid, and vitamin K, all the others are included.
Figure 9. Statistics on multiple nutrient supplement products in registered health foods
Analysis:
It can be seen from the number of nutrient supplements in the product that most of the products supplemented with multiple (three or more) nutrients, and the single nutrient supplement products are mainly several common nutrients, such as calcium, magnesium, iron, and zinc , Vitamin D, vitamin C, etc., and the two nutrient supplement products are mostly a combination of calcium and vitamin D.
6. Summary
Until June 30, according to the statistics released by the Food e
valuation Center of SAMR, there are o
nly 2 im
ported health foods in 2020. In fact, not o
nly two products have obtained the registration certificate of im
ported health food in 2020. Due to the centralized processing of information release (slightly delayed), some products completed in the first half of the year are not within the scope of this statistics. For example, the Food e
valuation Center of SAMR issued 30 im
ported health food filing vouchers on 3 July. Judging from the approval time of these 30 filing vouchers, products obtained filing vouchers every month. Foodmate will co
ntinue following the filling status.
COVID-19 epidemic have an impact on all sectors of society in 2020. In terms of applications for health food related products, im
ported health food may have the greatest impact. The situation of foreign epidemics is not optimistic, and the certification materials required for im
ported health food require foreign government agencies and the stamp of the Chinese Embassy, which will extend the application time due to the impact of the epidemic.
The above is the summary analysis of the current im
ported registered health foods by Foodmate. If you have any doubts or needs related to health food registration and filing, please leave a message to discuss or co
ntact us (010-68869850).
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net