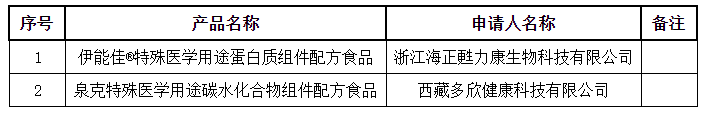
The National Medical Products Administration has released the approval letter (decision letter) for special medical use formula food on September 8, 2020 to receive the information
On September 8, THE State Medical Products Administration issued the approval letter (decision letter) for formula food for special medical use, which involves two products: yinengjia® protein compo
nent formula food for special medical use and quanke carbohydrate compo
nent formula food for Special medical use.
9月8日,国家药品监督管理局发布特殊医学用途配方食品批件(决定书)待领取信息,其中涉及伊能佳®特殊医学用途蛋白质组件配方食品、泉克特殊医学用途碳水化合物组件配方食品两种产品。
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net