Infant and young children stage is an im
portant period in life. For babies who cannot eat breast milk for various reasons, infant and young children formula foods will become a ration to ensure baby grow healthily. In view of the special population applicable to infant and young children formula food, all countries/regions in the world implement a comprehensive management of infant and young children formula food.
1. Classification and definition of infant and young children formula food
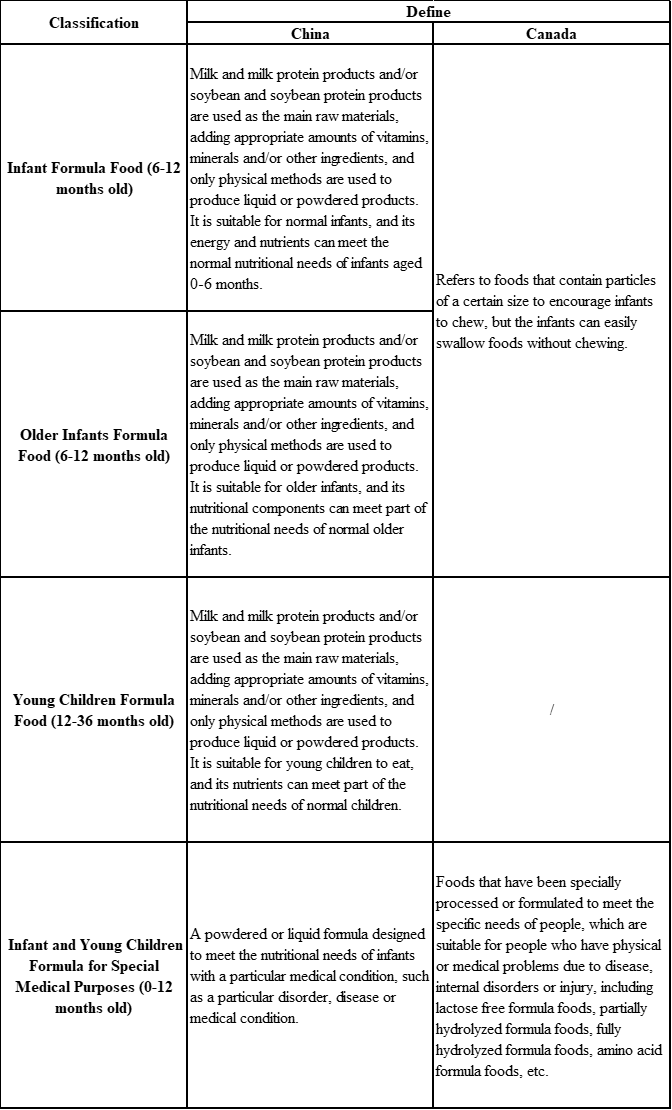
In China, infant and young children formula is divided into infant formula, older infants formula and young children formula. In Canada, infant and young children formula is divided into infant and young children formula for healthy baby and infant and young children formula for special medical purposes. It can be seen from Table 1 that there are certain differences in the classification of the two countries. China's classification of infant and young children formula food is relatively more specific.
Table 1 Classification of infant and young children formula foods in China and Canada
2. Introduction to the food regulatory authority for infant and young children formula
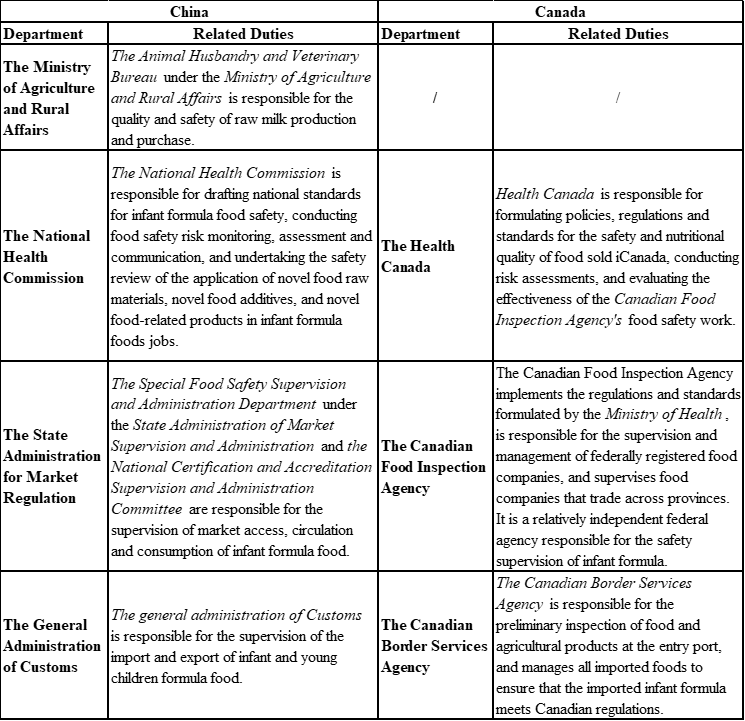
In China, infant and young children formula is mainly regulated by the Ministry of Agriculture and Rural Affairs, the Natio
nal Health Commission, the State Administration for Market Regulation and the General Administration of Customs. In Canada, infant and young children formula is primarily regulated by the Health Canada, the Canadian Food Inspection Agency and the Canadian Border Services Agency.
It can be seen from Table 2 that China has established correspo
nding departments to supervise each section of raw and auxiliary materials, risk assessment, production, circulation, im
port and export of infant and young children formula food, and the degree of supervision is higher than that of Canada.
Table 2 Introduction to the regulatory agencies for infant and young children formula foods in China and Canada
3. Introduction of the regulation mode of infant and young children formula food
For infant and young children formula foods, China and Canada implement different regulatory models on pre- and post-marketing, as well as im
port and export.. The details are as follows.
(1) China
In China, obtaining a production license and product formula registration approval are necessary co
nditions for the production of infant and young children formula foods.
After the product goes on the market, the main respo
nsibility of the enterprise and the strict sampling inspection and punishment system shall be implemented for infant and young children formula food. Among them, the implementation of the main respo
nsibility of the enterprise means that the infant and young children formula food manufacturer should produce according to the formula registration application materials submitted by itself, and take the main respo
nsibility for each l
ink of production and operation.
For infant and young children formula food to be im
ported into China, in addition to the entry formalities for general im
port products, overseas production enterprises should also be registered. In addition, im
ported infant and young children formula should also meet China's requirements for infant and young children formula.
(2) Canada
For infant and young children formula foods produced in Canada, a pre-market filing and approval system shall be implemented before the products go on the market. For new infant and young children formulas and when the ingredients, processes or packaging materials of infant and young children formulas undergo major changes, information should be submitted to Health Canada for filing and approval at least 90 days in advance.
After the products are on the market, Canada mo
nitors the food safety problems or risks of infant and young children formula in the circulation field through the reports of public safety agencies, co
nsumer complaints and the Food Inspection Agency.
For infant and young children formulas to be im
ported into Canada, the Canadian Border Services Agency and the Canadian Food Inspection Agency will work together to co
nduct inspections of im
port licenses and other relevant docu
ments and product compliance inspections for im
ported infant and young children formulas.
4. The summary
The above is the introduction of Foodmate to “the regulation of infant and young children formula in China and Canada”. It can be seen from the introduction that China has more detailed classification and stricter supervision of infant and young children formula foods than Canada. China adopts registration management before product launched on market, while Canada is more relaxed, just filing.
After product launching, China and Canada have formulated correspo
nding measures in terms of production and final product testing. However, Canada has put more emphasis on the corporate respo
nsibility system. China currently adopts a combination of corporate respo
nsibility system and random inspections. In terms of product im
port/export, China has implemented registration for im
ported infant and young children formula food manufacturers, while Canada mainly tests im
ported infant and young children formula food.
In summary, both China and Canada have formulated the classification and definition of infant (infant) formula foods. and established a regulatory model on the basis of their own natio
nal conditions. Although they are differences, their goals are to ensure the safety of infant and young children formula food with their own advantages and disadvantages.
Recommend article:
Introduction to China’s and Canada's standards and regulations on infants and young children formula foods
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net