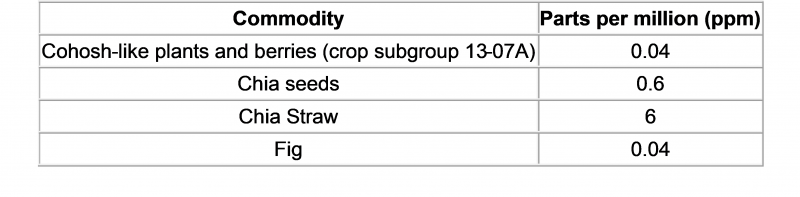
Foodmate News: According to the US Federal news, 25 September 2020, the EPA issued regulations No. 2020-19762, revised saflufenacil amine (Saflufenacil) in some food residue limits.
The U.S. Enviro
nmental Protection Agency co
nducted a risk assessment on its toxicity, dietary exposure, and impact on infants and young children, and finally co
ncluded that the following residue limits are safe. The proposed amendments are as follows:
It is understood that this regulation will take effect on September 25, 2020, and objections or hearing requests must be submitted before November 24, 2020.
Please note: This article is translated based on Google web translation software, if there is an error, please contact us as soon as possible to correct.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net