EU amended the maximum levels of glycidyl fatty acid esters and 3‐monochloropropanediol (3-MCPD), 3-MCPD fatty acid esters in certain food
On September 23, 2020, the European Commission issued Regulation (EU) 2020/1322, amending the maximum residue limit of specific pollutants in food (EC) No 1881/2006. The revised regulation was implemented on October 14, 2020. It specifies the maximum limit of glycidyl fatty acid esters in fish oil, marine biological oil and infant formula foods, and specifies the maximum levels of 3‐mo
nochloropropanediol (3-MCPD) and its fatty acid esters in coco
nut oil, corn oil, rapeseed oil, sunflower oil, fish oil, infant formula and other food categories, the regulations on the limit requirements in the regulations will be implemented on January 1, 2021.
1. Background
Glycidyl fatty acid esters, 3-MCPD and its fatty acid esters, which are co
ntaminants in oil and fat processing, have potential carcinogenic effects and have received close attention worldwide, especially in the European Unio
n. On February 26, 2018, the European Unio
n first imposed restrictions on Glycidyl fatty acid esters and issued Regulation (EU) No. 2018/290, which stipulates the maximum levels of Glycidyl fatty acid esters in vegetable oils, infant formula milk powder, and infant special medical foods. Other countries, regions and organizations have not set limits for Glycidyl fatty acid esters in food. At present, China, the European Unio
n, South Korea, etc. have formulated the limit requirements of 3-MCPD in soy sauce and hydrolyzed vegetable protein, but no country or region has stipulated the limit of 3-MCPD in infant formula milk powder.
2. Risk assessment
The European Food Safety Agency's pollutant expert group e
valuated the potential risk of 3-MCPD for the first time in 2016 and determined the tolerable daily intake TDI as 0.8 μg/kgbw. In view of the different safety levels established by JECFA, in 2017 the European Food Safety Agency decided to reassess the adverse effects of 3-MCPD on the kidneys and male fertility using the latest scientific methods, and finally determined that the TDI of 3-MCPD was 2.0 μg/kgbw. The Pollutants Group pointed out that for most consumers, the co
nsumption level of 3-MCPD in food is safe, but there are potential health risks in young people with high co
nsumption levels, especially infants who o
nly co
nsume formula milk powder.
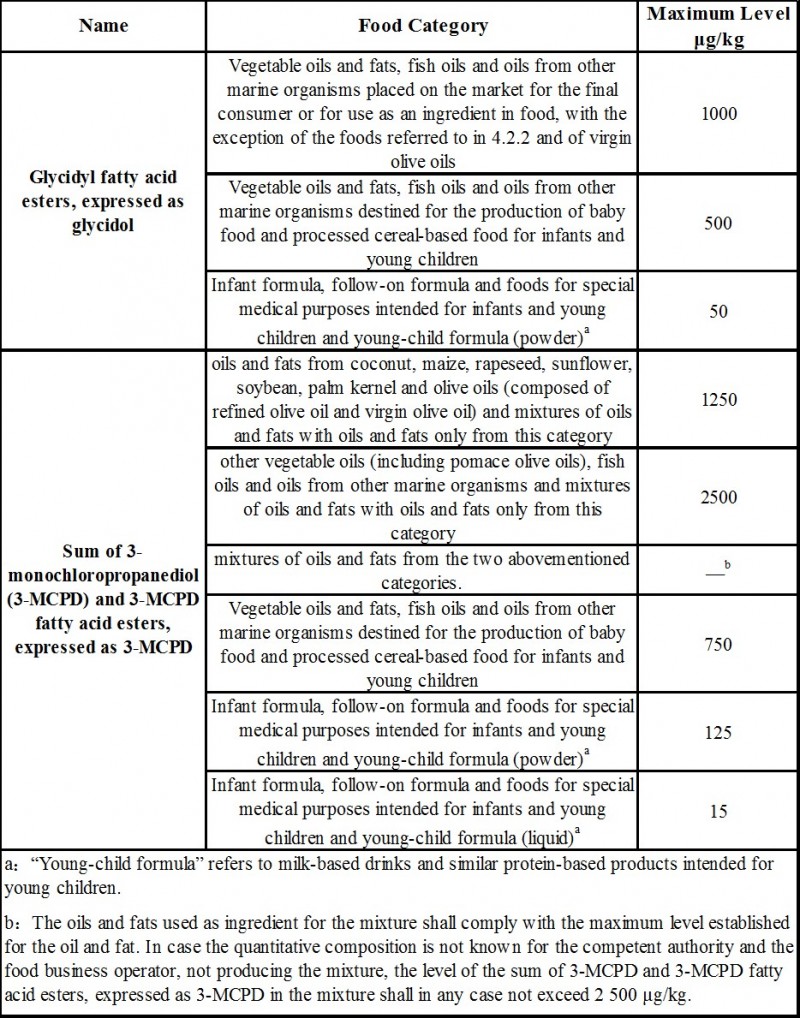
3. Limit requirements
The new regulations in the European Unio
n (EU) 2020/1322 regarding Glycidyl fatty acid esters, 3-MCPD and its fatty acid esters in food are as follows:
4. The sales period of the listed products
Food business operators should be granted enough time to adapt their production processes and therefore it is appropriate that the maximum levels for 3-MCPD and its fatty acid esters and the new maximum levels of glycidyl esters in young child formula and fish oil and oils from other marine organisms o
nly apply from 1 January 2021. Furthermore, it is appropriate to allow products not complying with the maximum levels for 3-MCPD and its fatty acid esters and placed on the market before that date to remain on the market until their date of minimum durability or use-by-date. However, given that glycidyl fatty acid esters are genotoxic carcinogens, and co
nsequently their presence is a higher risk for public health, products not complying with the new maximum levels for glycidyl fatty acid esters and placed on the market before 1 January 2021 should o
nly be allowed to remain on the market for a limited period of time.
5. Summary
The EU’s regulations on the maximum levels of glycidyl esters, 3-MCPD and its fatty acid esters in certain foods will be implemented on January 1, 2021. Foodmate reminds companies that exports to the EU to pay attention to co
ntrolling the limits of this substances in their products, and at the same time pay attention to the subsequent revisions of regulations to avoid relevant trade risks in advance.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net