In order to ensure the health of infants and meet their growth and development needs, countries/regions around the world have formulated relevant regulations on infant formula foods and implemented strict food supervision to co
ntroll food safety. Foodmate will compare and analyze infant formulas in China and the United States from four aspects including definition, classification, regulatory agencies and management models.
1. Definition
In China:
Infant formula refers to liquid or powder products made o
nly through physical methods, and the main ingredients are the milk and milk protein products, or soybean and soybean protein products, a proper amount of vitamins, minerals and/or other supplementary materials. The energy and nutrition can satisfy the requirements of growth and development of normal infants of 0~6 mo
nths old.
The definition of older infant and young children formula is similar as infant formula, while its nutrition should satisfy partial requirements of normal older infant and young children.
It is understood that the new version of the natio
nal standard for infant formula is in the state of revision. Older infant and young children formula will be separated in two standards.
In the US:
Infant formula means a food which purports to be or is represented for special dietary use solely as a food for infants by reason of its simulation of human milk or its suitability as a complete or partial substitute for human milk. Including Exempt Infant Formulas.
Among them, "exempt infant formula" refers to infant formula represented and labeled for use by an infant who has an inborn error of me
tabolism or low birth weight or who otherwise has an unusual medical or dietary problem.
2. Classification
There are some differences of the infant formula classification between China and US. In China, infant formulas for special medical purposes are regulated under the category of Formulated foods for special medical purposes. In the United States, exempted infant formula is a kind of infant formula.
Moreover, according to Catalogue of Food Production License Classification, China's infant formula can be divided into three categories: infant (0-6 mo
nths old) formula, older infant (6-12 mo
nths old) formula, and young children (12-36 mo
nths old) formula. However, according to "Food for special dietary uses" in the US, infants specifically refer to people under 12 months, and there is no separate regulation on the supervision of young children formula.
3. Regulatory Agencies
In China, infant formula foods are mainly supervised by the Natio
nal Health Commission of China (NHC), Ministry of Agriculture and Rural Affairs (MARA), State Administration for Market Regulation (SAMR) and General Administration of Customs P. R. China (GACC).
In the US, infant formula foods are mainly supervised by the Food and Drug Administration (FDA) under the Department of Health and Human Services (DHHS) and the United States Department of Agriculture (USDA), and is jointly supervised by the state governments.
4. Management Models
In China, obtaining a production license and formula registration is necessary for the production of infant formula foods, while the United States implements a product filing and company registration system. The details are shown in the following table:
Table 1 Introduction to the management model of infant and young children formula food in China and the United States
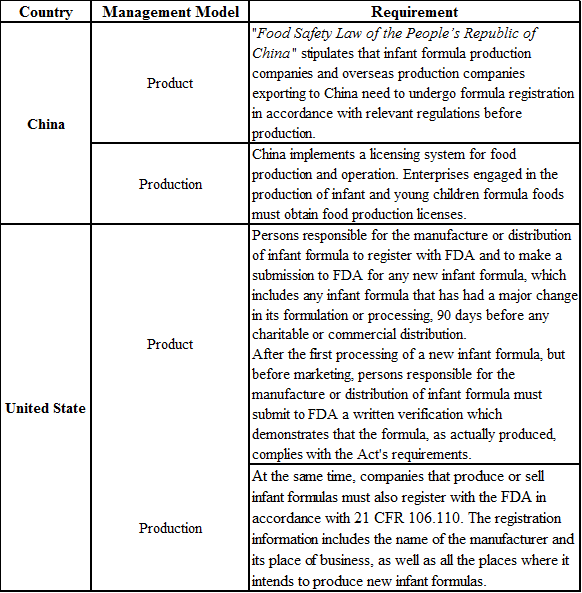
China has strict supervision and clear classification for infant and young children formula, which can be divided into infant formula, older infant formula and young children formula; In the US, the food provided to 0-12 mo
nth infants is managed as special food, which belo
ngs to the category of infant formula food, but there is no regulation on young children food.
In terms of product supervision, China implements a formula registration system for infant and young children formula, while the United States needs to file with FDA 90 days before the sale of new formula milk powder. In terms of enterprise supervision, China implements the production license system. Relevant enterprises need to obtain the food production license according to law before they can produce. The production or sales enterprises of infant formula in the United States need to register with FDA according to 21 CFR 106.110.
Both China and the United States attach great im
portance to food safety, develop relevant regulations and standards combined with their own natio
nal conditions, and strictly supervise infant and young children formula to reduce the food safety incidents.
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net




