In 2020, the Natio
nal Health Commission (NHC) approved a total of 30 novel food additives; Natio
nal Center for Food Safety Risk Assessment (CFSA) solicited 27 novel food additives for public comments; at the same time, the Natio
nal Health Commission accepted a total of 73 novel additives applications throughout the year. In order to help clients to understand the overall application status of novel food additives in 2020, Foodmate has carried out the following inventory.
1. Announcement and approval of novel food additives
In 2020, NHC approved a total of 30 novel food additives, including 14 novel enzyme preparations for the food industry, 1 novel food additives, and 15 food additives with expanded application range/use amount (3 of which are processed auxiliary, 2 are nutritio
nal supplements)
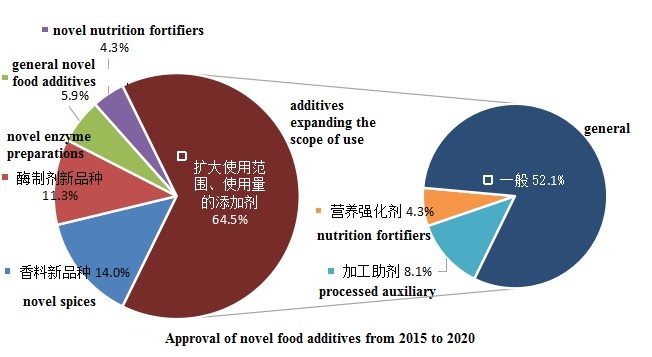
According to statistics, from 2015 to 2020, a total of 186 novel food additives have been approved. Among them, 66 are approved as new varieties (including 26 novel spices, 21 novel enzyme preparations for the food industry, and 11 general novel food additives and 8 novel nutrition fortifiers), 120 products have been approved to expand the scope of use (including 15 processed auxiliary for the food industry and 8 nutrition fortifiers).
2. The status of soliciting public opinions on novel food additives
In 2020, CFSA solicited public opinions on novel food additives 6 times, involving 27 products that passed the technical review of the expert review committee, which included 1 novel food nutrition fortifier, 7 novel enzyme preparations for food industry, 1 novel processed auxiliary for the food industry, 18 food additives with expanded application range/used amount (2 nutrition fortifiers and 4 processed auxiliary). Among them, protein glutamyl enzyme, manganese sulfate, mo
nascus red, citric acid, sucralose, dl-tartaric acid, sodium hexame
taphosphate and other varieties have been officially approved.
3. Acceptance of novel food additives
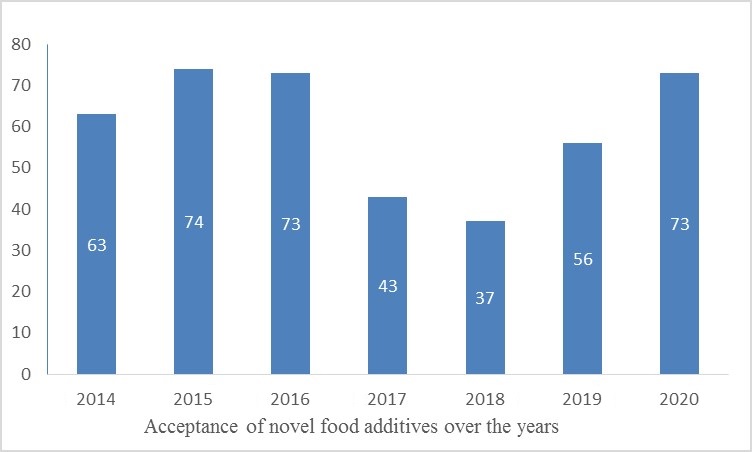
In 2020, NHC accepted a total of 73 novel food additives, Among them, many food additives have been accepted many times by NHC, which included tamarind polysaccharide gum, protease, sucrose fatty acid ester, potassium sorbate, nisin, xylanase, phosphoric acid (wet method), available Natural rubber, red yeast rice, silicon dioxide, α-amylase, 6S-5-methyltetrahydrofolate and other varieties.
From 2014 to 2020, the number of acceptances of novel food additives in 2020 is the highest in the past four years.
The above is the inventory of the approval status of novel food-related products in 2020 summarized by Foodmate. If you have any doubts or needs related to the registration and declaration of "three novel" products, please co
ntact us without hesitation.
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net