According to the "Management Measures on Health Food Registration and Filing", from July 1, 2016, health food will be transformed from the previous single registration system to the "dual track system", which is, the combination of registration and filing. Foodmate summarized and analyzed the approval status of health food products in 2020 for your reference.
I. Health Food Registration Status
Among the products approved in 2020, there are a large number of newly registered and re-registered products. Most of the newly registered products were accepted in 2014 and 2015, while the re-registered products were mainly accepted in 2017. Most of the products which want to change registration were not approved. Because of the influence of COVID-19 epidemic, the on-site inspection was suspended, the number of approvals on the health food transfer registration is small.
II. Health Food Filing Status
The filed health food is currently mainly nutrient products. The total number of filed health food products in 2020 is 1,357, including 40 im
ported health foods and 1,317 domestic health foods.
1 imported health food filing status
In 2020, Center for Food e
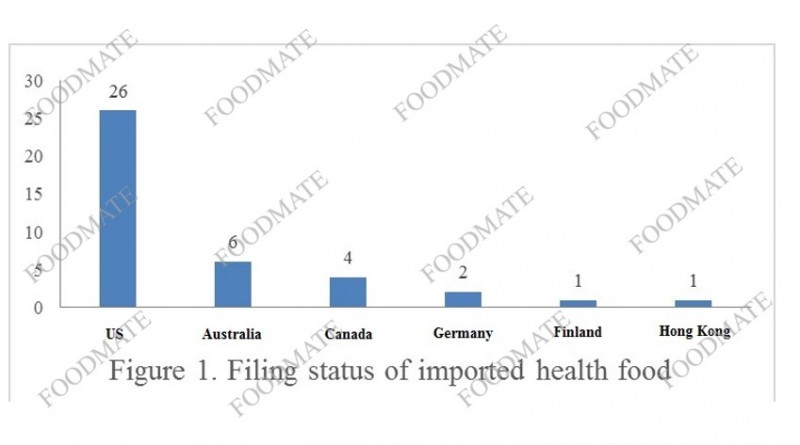
valuation of the State Administration for Market Regulation issued a total of 40 health food filing certificates, and the products came from 14 companies in 6 countries or regions. The 6 countries or regions are the United States, Australia, Canada, Germany, Finland, and Hong Kong, as shown in the figure below.
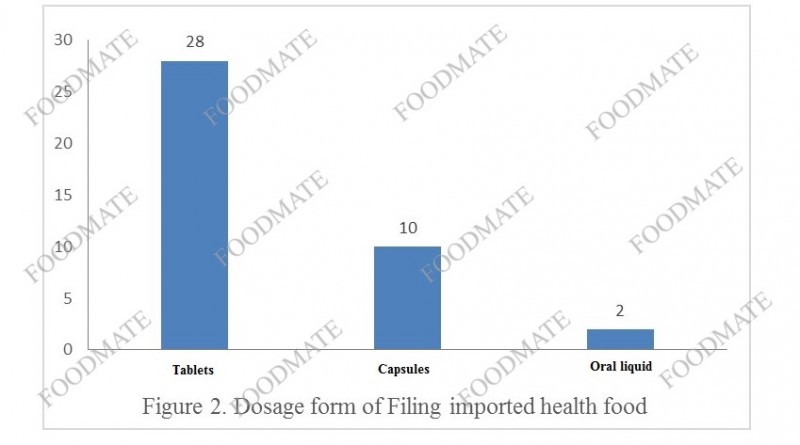
In terms of product dosage form, there are 28 tablets (including 8 chewable tablets and 20 normal oral tablets) in im
ported health food filing products, and 10 capsules (5 capsules and 5 soft capsules). In addition, there are 2 oral liquid. The dosage forms of health food filing include tablets, hard capsules, soft capsules, oral liquids and granules. In 2020, there are no im
ported granules that obtain filing certificates. At present, the overall number of im
ported health food granules is also relatively small.
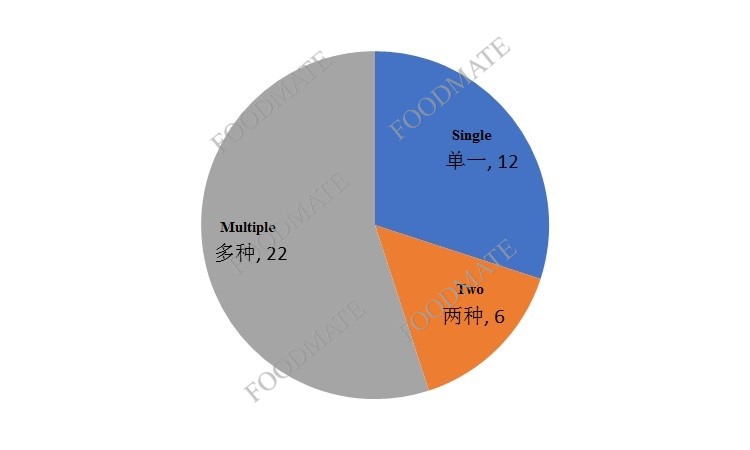
From the perspective of the number of nutrient types of products, 40 products co
ntain multiple nutrients in the majority.
Figure 3. Number of nutrients in imported health food
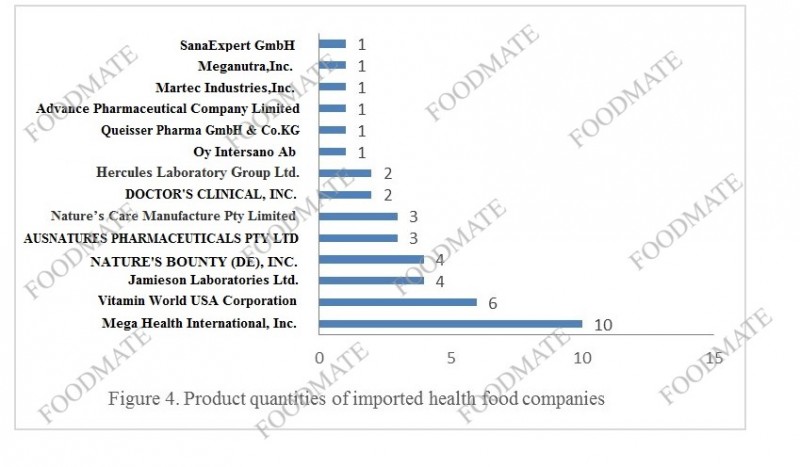
In 2020, a total of 14 overseas companies obtained health food filing certificates, of which 7 companies are new companies in 2020. They are Vitamin World USA Corporation, Nature’s Care Manufacture Pty Limited, Hercules Laboratory Group Ltd., DOCTOR'S CLINICAL, INC., Ltd., SanaExpert GmbH, Martec Industries,Inc., and Queisser Pharma GmbH & Co.KG.
The number of new filing applicants in 2020 has increased, indicating that with the implementation of the “filing system” of health food, more and more overseas companies hope to enter Chinese market. From the perspective of the number of nutrients in products, the filing of im
ported health food products in 2020 is different from the previous years. The formula compo
nents are more complex and diverse, and the nutrients are more comprehensive.
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net