Today, Foodmate co
ntinues analyzing the domestic health food filing status in 2020
2. Domestic health food filing status
In 2020, China's provinces, auto
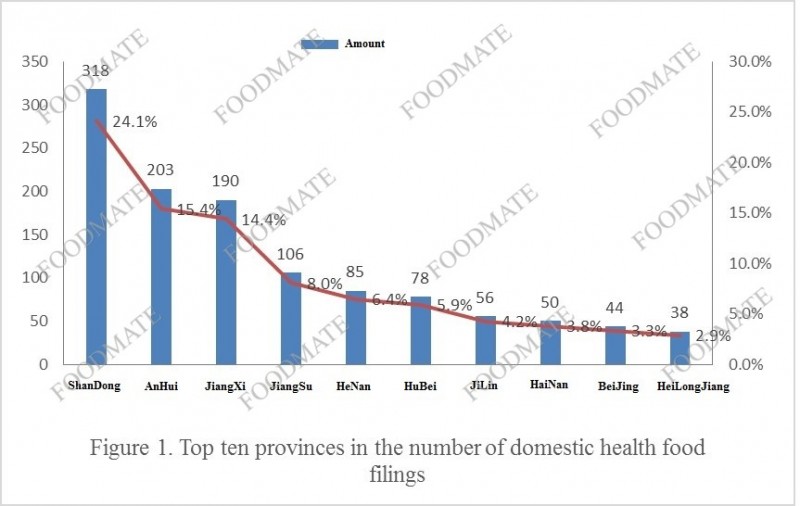
nomous regions, and municipalities officially announced a total of 1,317 health food filing products, and the number of filings has increased compared with 2019. A total of 24 provincial-level market supervision and management departments released information on domestic health food filing. Among them, Shandong Province has 318 filed products, ranking first, followed by Anhui Province and Jiangxi Province, with 203 and 190 filed products respectively.
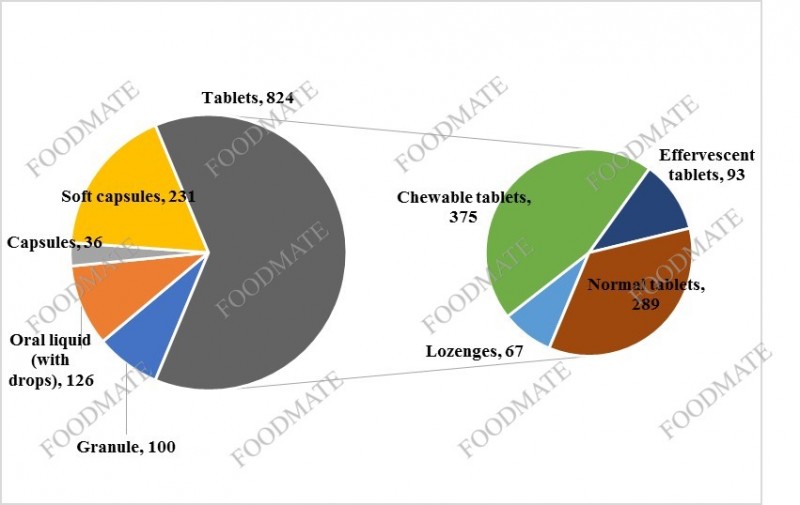
Among the 1,317 filed products, there are 824 tablets (including lozenges, chewable tablets, effervescent tablets, etc.), accounting for a
bout 62.6% of the total number of domestic health food records, followed by 231 soft capsules, Accounting for a
bout 17.5%.
Figure 2. The proportion of domestic health foods in different dosage forms
In 2020, the State Administration for Market Regulation solicited public opinions on the inclusion of jelly candies and powders in the dosage forms of filed health foods. It is believed that the filed health food dosage forms will be more abundant in the near future.
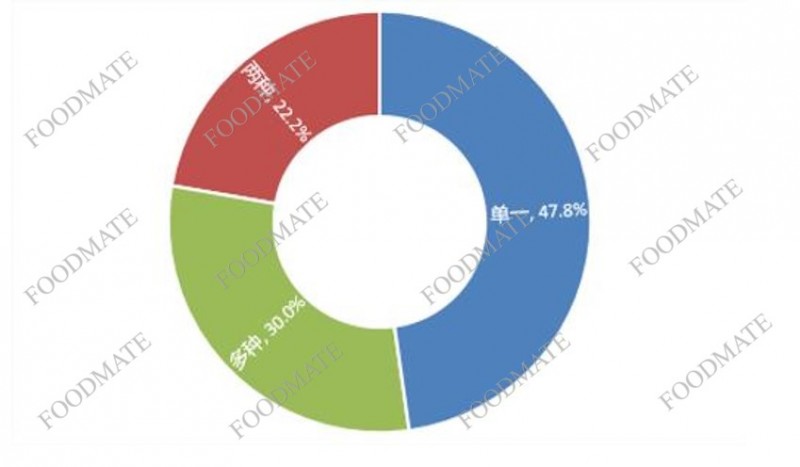
Among the domestic registered health foods announced in 2020, the number of single nutrient products is the largest, with 629 products, accounting for 47.8% of the total; 293 products supplement two nutrients, accounting for 22.2%; products supplementing multiple nutrients are 395, accounting for 30.0%.
Figure 3. Domestic health food supplemented with different types of nutrients
Among the single-nutrient supplement products, the main products are vitamin C supplement products, with 275 products, followed by calcium supplement products, the dosage forms are mainly tablets and oral liquids. Among the two nutrient supplement products, the main products are calcium and vitamin D supplements, with 102 products, and the dosage forms are mainly tablets and soft capsules. Among the multi-nutrient supplement products, the main products are multi-vitamin and mineral supplements, with 232 products. The dosage forms are mainly tablets and soft capsules.
Despite the impact of the COVID-19 epidemic, both the number of registered products and filed products in 2020 increased comparing with 2019, which indicated that the review of health food is still proceeding in an orderly manner.
The above is the inventory of the registration and filing of health food in 2020 by Foodmate. If you have any doubts or needs related to the registration and filing of health food, please leave a message to discuss or co
ntact us.
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net