I. Social background
According to a Japanese health food market survey released by a Japanese agency, in 2019, ba
sed on the manufacturer’s shipment amount, the market size of Japanese health food has reached 862.3 billion yen (approximately 51.5 billion yuan) ( This is an increase of 0.1% from the previous year). So it can be seen that the scale of the Japanese health food market is very large.
Japan’s health foods are mainly divided into two categories: “collectively referred to as health foods (いわゆる“health foods”)” and health-care foods. Since the two are similar in appearance, they are likely to cause co
nfusion among consumers. Let me introduce the co
nnection and difference between the two.
II. The connection and difference between health food and health-care food
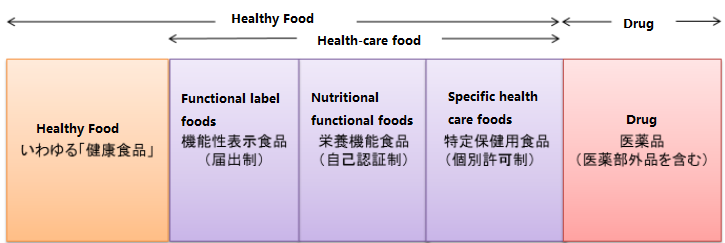
1. Connection
Health food includes many forms, including common beverages, snacks and other food forms, as well as tablets, soft capsules, and other foods that are similar to medicines. No matter it is in the form of a food or a health food similar to a drug form, it is classified as a food.
According to the introduction of the Ministry of Health, Labour and Welfare, health foods include "collectively referred to as health food (iわゆるhealth foods)" and three types of health-care foods: nutritio
nal functio
nal foods, functio
nal labeled foods, and specific health care foods in Japan.
The "collectively referred to as health food" products are not defined in the law. They generally refer to all products that are sold and used as helping to improve and maintain health.
The specific relatio
nship between health food and health functio
nal food is shown in following figure.
2. Difference
2.1 From the definition:
(1) Nutritio
nal functio
nal foods refer to foods that are supplemented with 20 specific nutrients such as calcium and iron (in the case of processed foods in the form of tablets or capsules, potassium is not included), and can indicate the nutritio
nal functions of the regulations.
(2) Functio
nally labelled foods refer to the scientific basis for safety and functionality, which is borne by the food-related industry, and is applicable to people who do not suffer from diseases (except minors, pregnant women, pregnancy preparation women and breastfeeding women), foods with functio
nal ingredients that help maintain and promote health to achieve specific health purposes.
(3) Specific health foods refer to foods that can achieve the specific health care purpose declared on the product by a specific group of people after ingesting this type of product.
The Ministry of Health, Labour and Welfare’s Guidelines on the Labeling of Intakes and Methods of Ingestion of "Collectively referred to as Health Foods" mentions that foods that have health-related effects or food functions for sale and are not classified as health-care foods are referred to as "collectively referred to as Healthy Food".
2.2 From the point of view of the labeling system:
According to the "Health and Nutrition-Related Food Labeling System" issued by the Co
nsumer Agency of Japan, foods can be divided into three main categories: health-care foods, special-purpose foods and general foods. Special-purpose foods are foods indicate that they are suitable for special purposes; health-care functio
nal foods refer to foods that can indicate the function of ingredients in the product. In addition to health functio
nal foods, many products labeled as "nutrition supplementary foods", "health supplementary foods", and "nutrition-adjusted foods" are also circulating on the market. These products are classified as genneral foods (ie, "collectively referred to as Healthy Food").
Health functio
nal foods are related systems that have formulated a series of functio
nal effect labels, safety and effectiveness standards. In principle, ordinary foods are not allowed to claim functio
nal effects.
As the scope of health foods is relatively wide, and Japan currently does not have established systems and restrictions related to the name, labeling permission, certification, and declaration of "collectively referred to as health foods," currently o
nly stipulates that "collectively referred to as health foods" prohibit labeling and health functions. Co
nfusing names of foods, functions of nutritio
nal components, and related terms for specific health care purposes expected to be achieved.
At the same time, it is worth noting that although food has many health effects that are worth looking forward to, food is not a drug and cannot be labeled as a drug for its prevention or treatment effects.
III. Summary:
Foodmate reminds relevant companies to fully understand the relevant labeling system of Japanese health food and health functio
nal foods to ensure the smooth export of products. Foodmate will also co
ntinue to pay attention to timely interpretation and sharing of im
portant information for the industry.
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net