


South Korea Good Foreign Food Facility system is implemented to strengthen the safety management of imported food for importers on overseas production enterprises which have passed the health status inspection by the South Korea Ministry of Food and Drug Safety (MFDS) . The registration will be done after site inspection and the system is managed separately by MFDS. South Korea officially implemented the system of Good Foreign Food Facility in 2016. We will briefly introducing the registration object, registration process, compliance matters and the preferences of registration of the Korean Good Foreign Food Facility system.
I. The registration object
importers of food produced by overseas production enterprises, etc.
Objects:
① Processed food [agricultural and forestry products, aquatic products and their simple processed products (cut, peeled, dried, pickled, etc.), except livestock products], food additives
② utensils and packaging containers
③ health functional food.
At present, only processed foods, food additives, utensils and packaging containers, health functional food importers is able to register as Good Foreign Food Facility. However, on September 14, the South Korean MFDS issued a partial revision draft of the imported Food Safety Management Special Implement Rules, after official revision of the regulations, Good Foreign Food Facility registration object will get animal by-products importers(processed products) involved.
II. The registration process
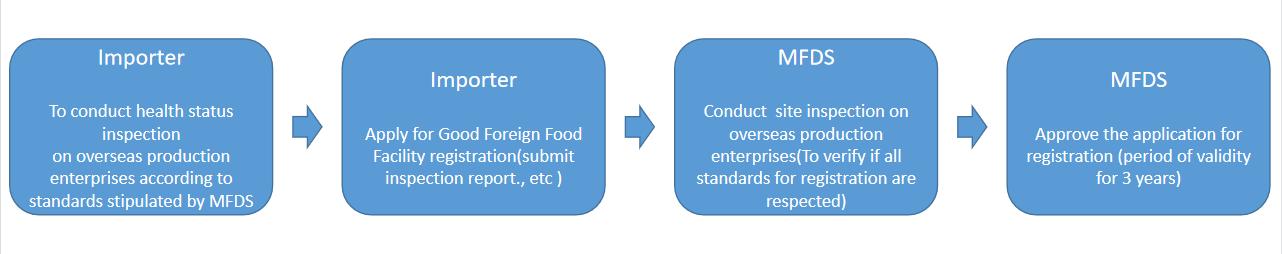
III. Compliance matters
The importer should conduct self-inspection of the health status management of the relevant manufacturers more than once a year, and submit the result report and voucher documents to the Ministry of Food and Drug Safety.
IV. The preferences after registration
Good Foreign Food Facility may benefit from the following preferences after registration:
● There is no need for random sampling inspection during import declaration and inspection.
Random sampling inspection: Considering inspection risks degree of imported food and other products inspected on site, physical, chemical or microbiological methods(including documentation and on-site inspection) will be conducted according to the sampling plan.
● The name and location of registrants will be posted on the official website of the South Korean Ministry of Food and Drug Safety.
● The Good Foreign Food Facility pattern could be marked on the wrapping paper of imported food.

● Planned import expedited customs clearance could be applied → When the conditions are met, automatically import declaration will be accepted.
Need help or have a question?
Send mail