

On December 11, 2022, U.S. Food and Drug Administration (FDA) issued a recall notice stating that Byheart is recalling five batches of infant formula milk powder because the product may be contaminated with Cronobacter sakazakii. The recalled products are as follows:
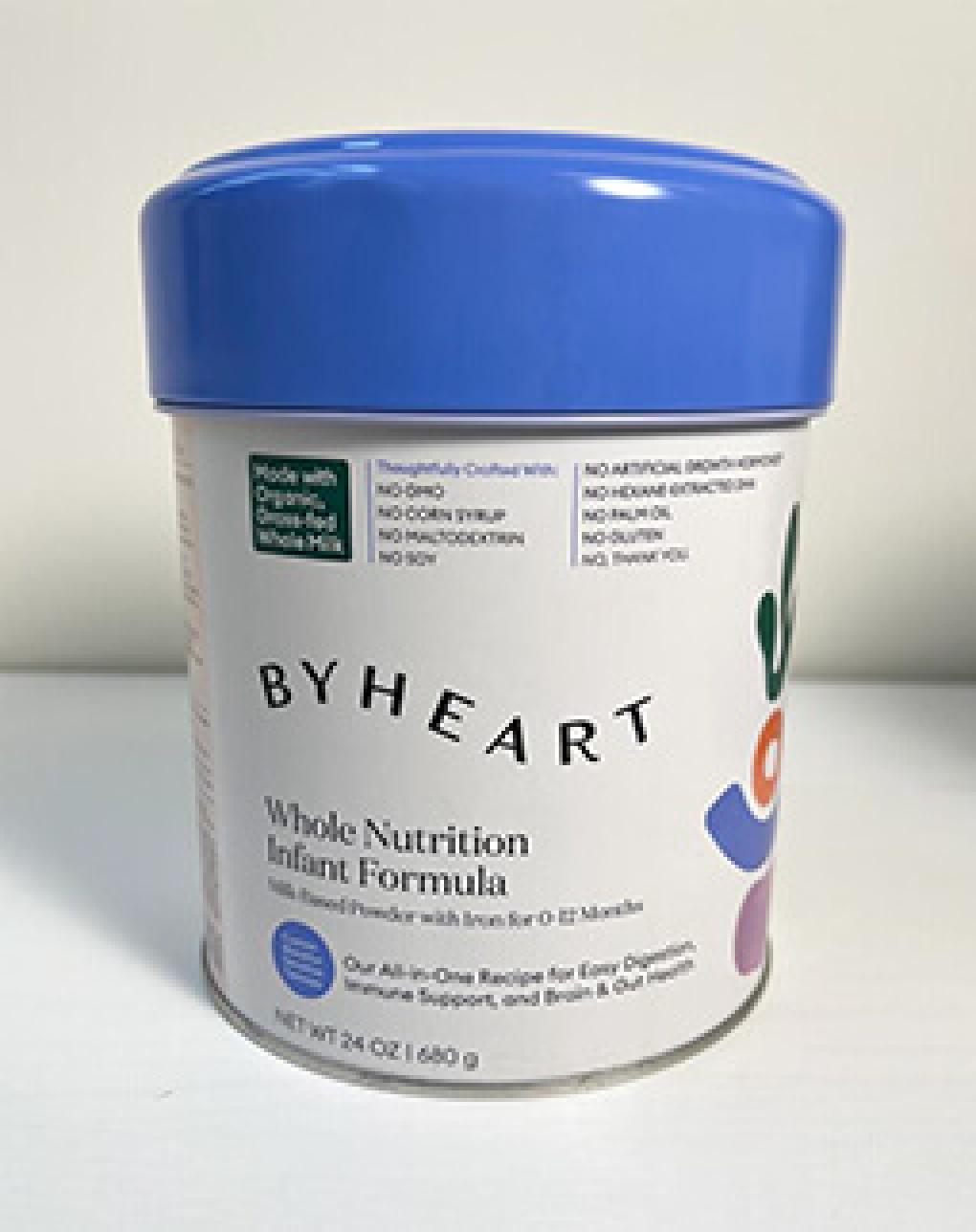
The product being recalled is ByHeart Whole Nutrition Infant Formula, Milk based Powder with Iron for 0-12 Months in 24 oz containers. The formula under voluntary recall was distributed directly to consumers in the U.S. and can be identified by the number on the bottom of the can. Recalled product batches are 22273 C1, 22276 C1, 22277 C1, 22278 C1, and 22280 C1 printed with use by 01 JAN 24 or 01JUL 24.
Currently, none of the distributed ByHeart product has tested positive for any contaminants. No consumer complaints received, to date, that would indicate any illness. The FDA advises consumers to return it to the store of purchase for a full refund.
Need help or have a question?
Send mail