


On 17 May 2023, the European Food Safety Authority (EFSA) issued its conclusions on the review of existing MRLS for dithiocarbamate. The main contents include:
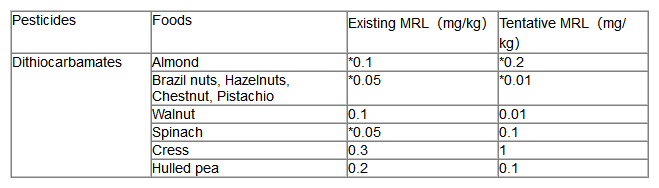
(1) In accordance with Article 12 of Regulation (EC)No 396/2005, EFSA has reviewed the maximum residues (MRLs) currently established at the European level for active substances for dithiocarbamate pesticides, Determination of Dithiocarbamates (including maneb, mancozeb, metiram, propineb, thiram and ziram), expressed as CS2;
(2) For animal substrates (meat, fat, liver, milk, and eggs), there are analytical methods that perform the proposed residue definitions based on converting methachlor or deisen zinc to CS2 and quantification by GC-MS with LOQs of 0.01mg/kg (expressed as CS2) and 0.03mg/kg, respectively. Dithiocarbamate (represented by CS2) and ETUs (common metabolites containing deisenian, mancozeb and manganese) are not expected to be high in animal commodities in the calculated tentative dietary intake. Therefore, a minimum LOQ value of 0.01mg/kg for CS2 and a risk assessment value of 0.01mg/kg for ETUs in all products of animal origin are recommended. It is important to stress that these MRLS and risk assessment values are only tentative, as the calculated dietary burden is expected to be underestimated, the extraction efficiency of the analytical methods performed is not demonstrated, and livestock feeding studies are not adequately supported by the data. Therefore, the resulting MRL and risk assessment values may need to be reconsidered once more data are available to bridge the gap in residues in plant and animal commodities;
(3) metabolite ETUs are classified as reproductive toxicity Class 1B under Regulation (EC)1272/2008 and meet the ED criteria for humans and may therefore require further consideration by risk managers. Some of the proposed changes are as follows:
Need help or have a question?
Send mail