

![[240410]507f645623e1323a58a2a8999b462d19.jpeg.jpeg [240410]507f645623e1323a58a2a8999b462d19.jpeg.jpeg](https://global.foodmate.net/file/upload/image/20240410/[240410]507f645623e1323a58a2a8999b462d19.jpeg.jpeg)
On 5 April 2024, the European Commission published Regulation (EU) 2024/1003, amending Regulation (EU) 2023/915 as regards maximum levels for the sum of 3-monochlorpropanediol (3-MCPD) and 3-MCPD fatty acid esters in infant formulae, follow-on formulae and food for special medical purposes intended for infants and young children and young child formulae. The specific amendments are as follows:
In Annex I to Regulation (EU) 2023/915, section 5, entry 5.3.3 is replaced by the following:
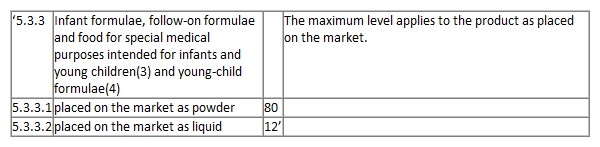
It is understood that this regulation shall enter into force on the 20th day of its publication in the Official Journal of the European Union. Applicable from 1 January 2025.
Learn more: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=OJ:L_202401003
Need help or have a question?
Send mail