

![[240528]672b1d2088773f1a8888290f268824dd.jpeg.jpeg [240528]672b1d2088773f1a8888290f268824dd.jpeg.jpeg](https://global.foodmate.net/file/upload/image/20240528/[240528]672b1d2088773f1a8888290f268824dd.jpeg.jpeg)
Recently, the United States FDA website updated the import alert measures (import alert), in which the relevant products of a Chinese company were automatically detained, the details are as follows:
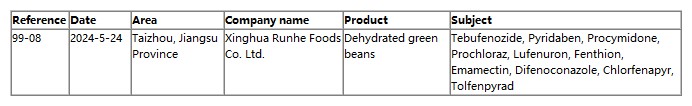
import alert is the FDA for the existence of potential risks of imported food in the customs clearance of a processing measure, for the import alert requirements of the enterprise / product, the FDA will be in the absence of inspection of the enterprise / product implementation of automatic detention (DWPE). Automatic detention does not mean that the export products do not meet U.S. import standards, was implemented “automatic detention” of imported goods, subject to FDA or FDA-approved laboratory inspection, and by the FDA in the local branch of the audit approval, the Customs and Excise Department will only be allowed to release the goods into the U.S. territory for sale.
Foodmate reminds the relevant export enterprises, in strict accordance with the requirements of the importing country for the export of products to ensure the safety of food and related products, to avoid the risk of export products are detained.
Need help or have a question?
Send mail