


Since October 7, 2023, the National Health Commission of China has approved five products containing two types of HMOs (Human Milk Oligosaccharides) substances - 2'-fucosyllactose and lacto-N-neotetraose, as new varieties of food additives for use in China, marking a new breakthrough in the field of food additives for HMOs. Due to their unique nutritional value and health benefits, HMOs have shown broad application prospects in areas such as infant formula. With the increasing number of new food additive varieties being submitted for approval in 2024, HMOs are undoubtedly becoming a hot topic of industry attention. Foodmate has sorted out the acceptance and approval status of HMOs as new food additive varieties.
1 Application Status of New Food Additive Varieties in China
From August 2016 to June 18, 2024, the National Health Commission (including the former National Health and Family Planning Commission) has received 33 applications for new food additive varieties of HMOs substances.
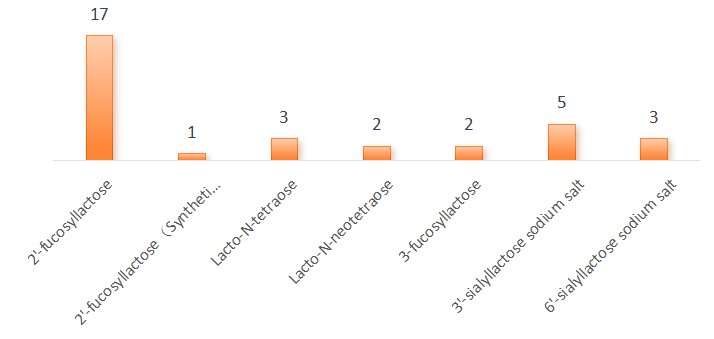
Figure 1.1: Summary of Acceptance Frequency of New Food Additive Varieties Containing HMOs
From the figure above, it can be seen that the acceptance frequency of 2'-fucosyllactose is the highest, which is also the most abundant in human breast milk. The acceptance frequencies of other HMOs varieties are relatively average.
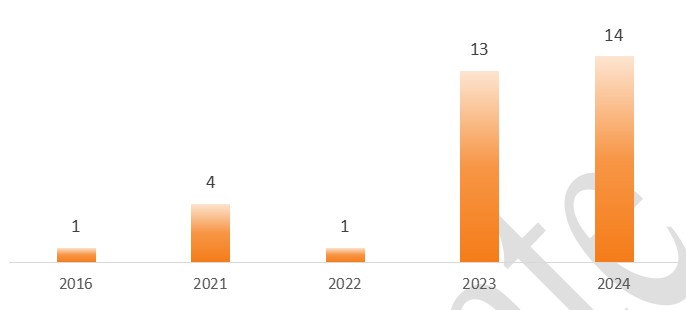
Figure 1.2: Annual Summary of Acceptances for New Food Additive Varieties Containing HMOs
From the figure above, it can be observed that in just the first half of 2024, the acceptance frequency of HMOs for 2023-2024 has already reached 82% of the total acceptances, reflecting the significant potential demand for HMOs in the Chinese market.
2 Approved Status
2.1 Approved in China
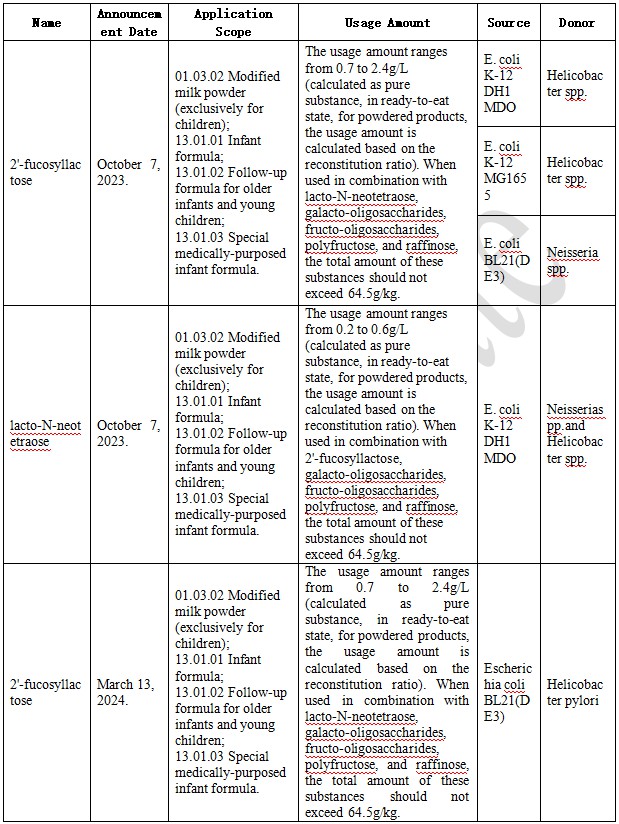
The announcement released on October 7, 2023, regarding 15 "New Food Ingredients, New Food Additives, and New Food-related Products" including peach gum, and the announcement released on March 13, 2024, regarding 23 "New Food Ingredients, New Food Additives, and New Food-related Products" including Dendrobium stem bulbs, both contain approval information for five products involving HMOs. A summary is provided below.
2.2 Approved in foreign countries
The approval forms of HMOs substances vary among different countries. In the United States, the approved usage information is released through the GRAS (Generally Recognized As Safe) notification. In the European Union, HMOs are authorized for use as novel foods. In Australia and New Zealand, HMOs are used by amending the corresponding standards in the Australia New Zealand Food Standards Code. The approval status in various countries/regions is summarized in the following table.
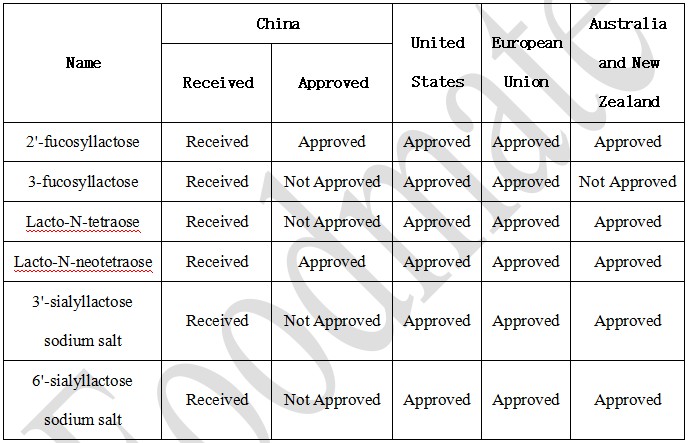
From the table, we can see that the HMOs substances that have been approved in the United States, European Union, and Australia and New Zealand have mostly been received for review in China, but only two substances have been approved for use.
3 Conclusion
Over time, it is expected that more HMOs substances will be approved in China to meet market demand. HMOs, as an important component in breast milk, are widely recognized for their unique nutritional value and functions. They will promote the upgrading and development of the infant formula food market, creating more opportunities for overseas manufacturers intending to export infant formula to China. Additionally, as consumers' understanding of healthy foods deepens, the application areas of HMOs will also be further expanded. Foodmate will continue to monitor domestic and international approval progress and provide timely and accurate information support for the industry.
Need help or have a question?
Send mail