

We already have introduced the classification and definition of formula food for infants and young children in Chinese mainland and Taiwan, China. In this article, Foodmate will compare the infant and young children formula from Chinese mainland with Taiwan, China from two aspects: regulatory agency and regulatory mode.
1. Introduction of infant and young children formula regulatory agency
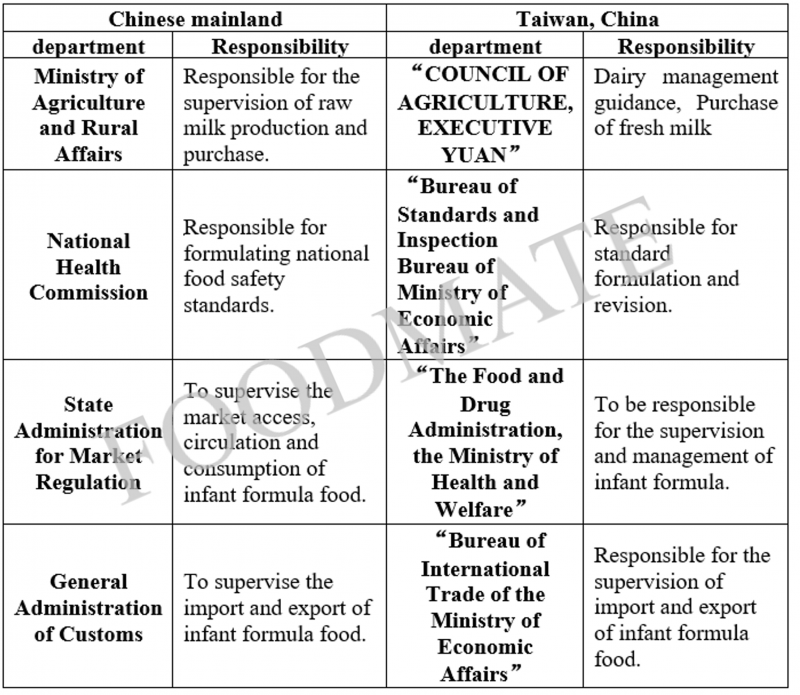
2. Introduction of supervision mode of infant and young children formula
When infant (infant) formula food are before and after put on the market and during import and export, the specific regulatory patterns in the Chinese mainland and Taiwan are as follows.
2.1 Chinese mainland
In China, infant and young children formula food products intend to sell in the Chinese mainland need to get formula registration certificate before put on the market and the manufacturer should obtain the production license. For the infant formula food to be imported into China, in addition to the general import procedures and product formula registration, the manufacturer of the overseas manufacturer shall also be registered.
After the products are put on the market, the enterprise's main responsibility and strict sampling and punishment system will be implemented for infant and young children formula. Among them, the implementation of the main responsibility of enterprises means that the infant and young children formula food production enterprises should produce according to the formula registration application materials submitted by themselves, and bear the main responsibility for all aspects of production and operation.

2.2 Taiwan, China
Infant formula produced in Taiwan,China need to submit relevant information for inspection and registration before they can be put on the market.For the infant formula to be imported into Taiwan,China, the import license and other relevant documents shall be inspected and the product compliance inspection shall be conducted.
After the products are put on the market, they shall be supervised and managed by the food and drug administration. Strict sampling inspection, verification and punishment system were carried out for infant formula.
3. Summary
To sum up, in terms of supervision, the Chinese mainland adopts the registration management of product formula before product put on the market, while Taiwan is relatively loose, just for filing. Both mainland China and Taiwan, China attach great to food safety, and both have formulated relevant standards and regulations for infant (infant) formula food based on their own premise. Although there are differences, their goals are to ensure the safety of infant (infant) formula food. Foodmate will continue to study the regulatory system of infant formula in different countries/regions, please look forward to!
Need help or have a question?
Send mail