


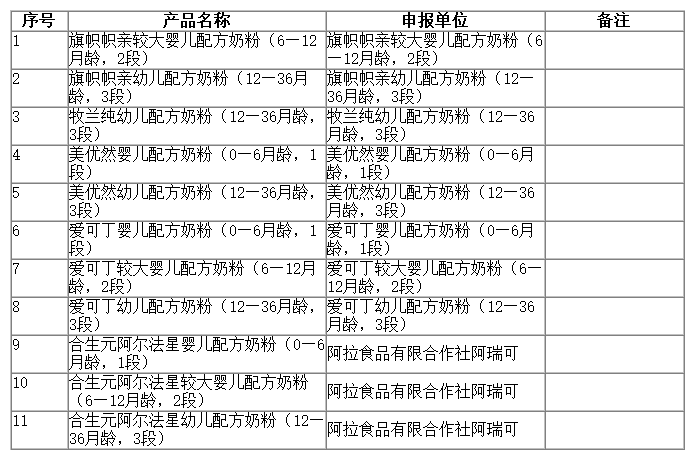
On May 25, 2023, the Center of Food evaluation of the State Administration for Market Regulation updated the list of approval document (decision) for the registration of infant formula product, which includes 11 kinds of infant/older infant/young children formula products, decision details as below: Qizhi Zhiqin follow-on formula (6-12 months, stage 2), Qizhi Zhiqin young children formula (12-36 months, stage 3).
国家市场监督管理总局食品审评中心发布2023年5月25日婴幼儿配方乳粉产品配方注册批件(决定书)邮寄信息,涉及旗帜帜亲较大婴儿配方奶粉(6—12月龄,2段)、旗帜帜亲幼儿配方奶粉(12—36月龄,3段)等11种婴幼儿配方乳粉类产品。
Need help or have a question?
Send mail