

Core Tip: On July, 2019, National Medical Products Administration (NMPA) announced 6 batches of the information on the registration of Infant Formula (approved), respectively on July 5, July 12, July 15, July 25, July 30 and July 31.
Global Foodmate summarised the published information of approved Infant Formula in July, 2019, 54 formulation alteration from 12 companies, 1 registration from 1 company.
The approval newly registered infant formula in July, 2019 is as follows:

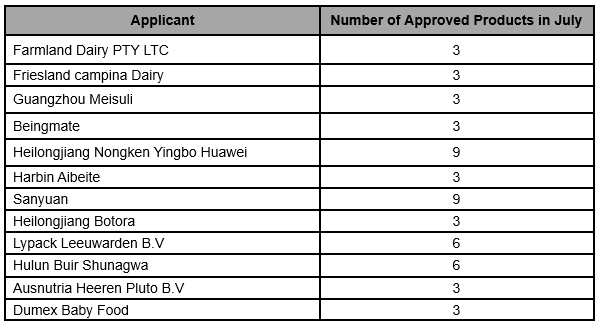
The situation of infant formula formulation alteration in July, 2019 is as follows:
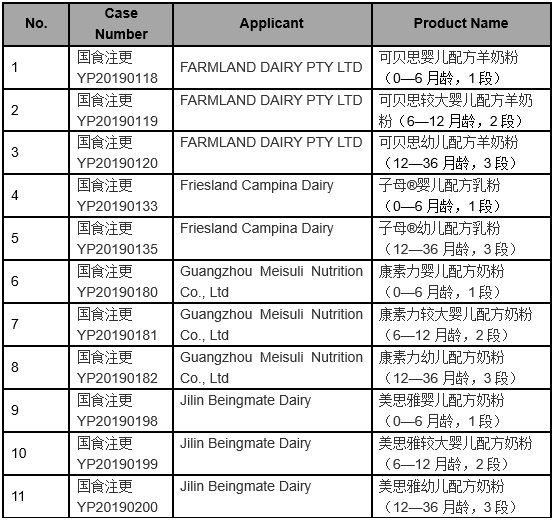
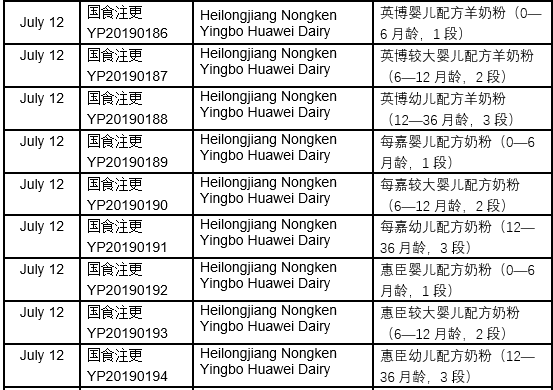
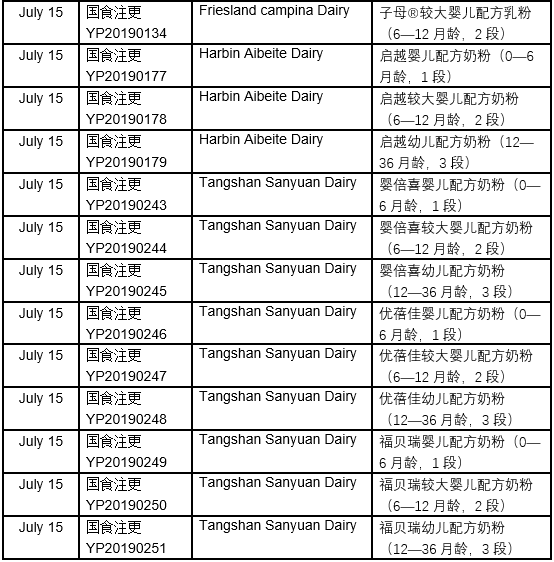
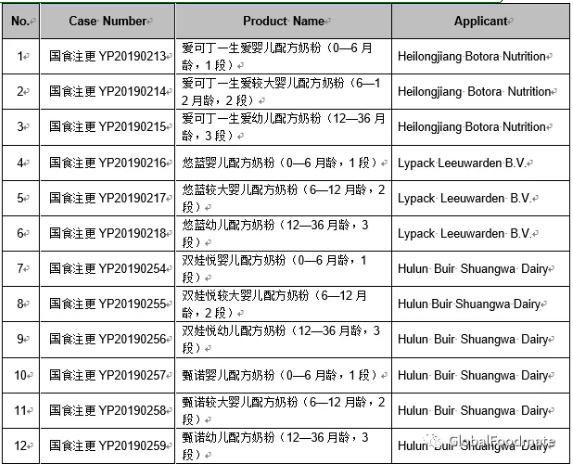
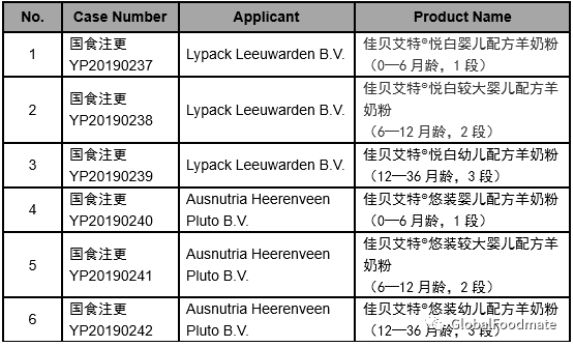
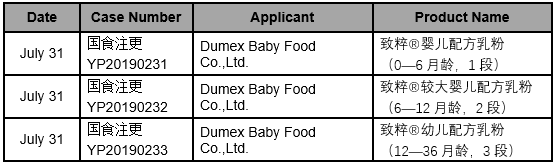
The detailed approval list of infant formula formulation alteration in July, 2019 is as follows:
Need help or have a question?
Send mail