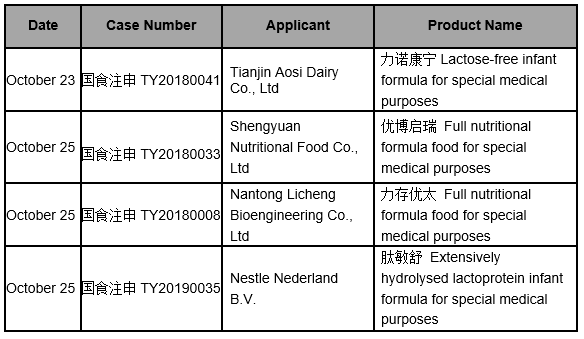
Core Tip: On October, 2019, National Medical Products Administration (NMPA) announced 2 batches of the information on the registration of Food for Special Medical Purposes (FSMP) (approved), respectively on October 23 and October 25.
Global Foodmate summarised the published information of approved FSMP in October, 2019, involving 4 formulas from 4 companies.
Please note: Original English article of Global Foodmate of Information Service and Business Department, please indicate the source from the Global Foodmate if reprint.
Global Foodmate Information Service Centre provides food standards & regulations research, labeling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net