NMPA | Approval document (Decision) for Infant Formula Milk Powder Product Formula Registration to Be Received on June 4, 2020
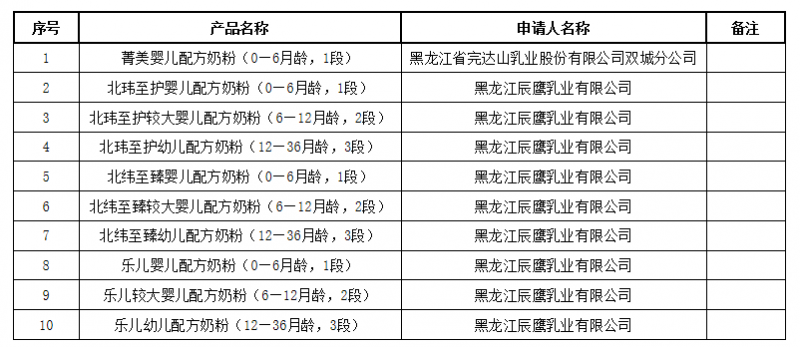
On June 4, the National Medical Products Administration issued the approval document (decision) for infant formula milk powder products formula registration to be received, including 10 products such as Jingmei infant formula milk powder (0-6 months old, Stage 1).
The details are as follows:
国家药品监督管理局发布2020年06月04日婴幼儿配方乳粉产品配方注册批件(决定书)待领取信息
6月4日,国家药品监督管理局发布婴幼儿配方乳粉产品配方注册批件(决定书)待领取信息,包括菁美婴儿配方奶粉(0—6月龄,1段)等10种产品。
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net