

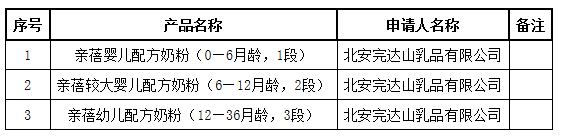
国家药品监督管理局发布2020年06月3日婴幼儿配方乳粉产品配方注册批件(决定书)待领取信息
6月3日,国家药品监督管理局发布婴幼儿配方乳粉产品配方注册批件(决定书)待领取信息,包括亲蓓婴儿配方奶粉(0—6月龄,1段)等3种产品。
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net
Need help or have a question?
Send mail