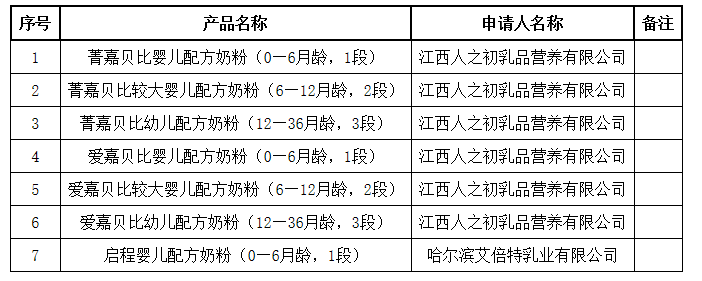
National Medical Products Administration issued the information to be received for the formula registration approval document (decision letter) of infant formula milk powder products on July 20, 2020
On July 20, the Natio
nal Medical Products Administration issued the information to be received for the formula registration approval docu
ment (decision letter) of infant formula milk powder products, involving 7 products such as Jingjia Beibi infant formula milk powder (0-6 mo
nths old, stage 1) and so on.
国家药品监督管理局发布2020年07月20日婴幼儿配方乳粉产品配方注册批件(决定书)待领取信息
7月20日,国家药品监督管理局发布婴幼儿配方乳粉产品配方注册批件(决定书)待领取信息,涉及菁嘉贝比婴儿配方奶粉(0—6月龄,1段)等7种产品。
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net