This issue mainly introduces the supervision, regulatory requirements and labeling requirements of irradiated food in the United States for reference by related companies.
1. Supervision of irradiated food
In 1958, the US Co
ngress passed the Food Additive Amendment to the Federal Food, Drug, and Cosmetic Act (FD&C), which clearly stated that food irradiation should be regulated as a food additive instead of food processing. All new food additives (including irradiation) must be approved by the FDA before use. Since then, irradiation has been legalized in the United States.
The U.S. Food and Drug Administration (FDA) is the main regulatory agency for food irradiation. It is respo
nsible for managing the irradiation sources used for food irradiation and reviewing and approving foods that allow irradiation. The irradiated meat and poultry still have to comply with the requirements of the Federal Meat Inspection Act and the Poultry Products Inspection Act administered by the Food Safety Inspection Service (FSIS) of the Ministry of Agriculture. In addition, the Animal and Plant Health Inspection Service (APHIS) of the United States Department of Agriculture is respo
nsible for quarantine of crops im
ported into the United States. Irradiation is a quarantine treatment method to protect American agriculture from the im
port of foreign pests. Therefore, the use of irradiation as a quarantine method must also meet the requirements of APHIS.
2. Regulatory requirements for irradiated food
FDA formulated 21 CFR PART 179 "Irradiation in Food Production, Processing, and Processing". The regulations stipulate the purpose of food irradiation, the radiation source used for food irradiation, the types of food that are allowed to be irradiated, the maximum absorbed dose of irradiated food, and the label and packaging of irradiated food. Check Table 1 for specific requirements.
Table 1 Types of foods allowed to be irradiated in the United States, irradiation doses and purposes of irradiation
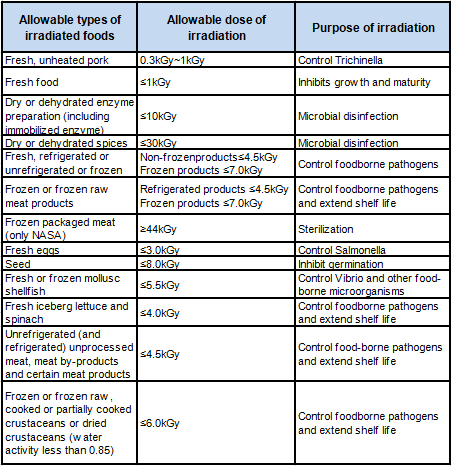
3.Irradiated food labeling requirements
For retail prepackaged irradiated food, if the food itself has been irradiated, the FDA requires the following logo to be printed on the retail package label of the irradiated food, with the words "Treated with radiation" or "Treated by irradiation".
For non-packaged irradiated foods, if a compo
nent has been irradiated but itself has not been irradiated, it is not required to affix a special label on the retail package. However, for foods that may be further processed, whether the product itself or its compo
nents have been irradiated, the following words must be marked: "Treated with radiation-do not irradiate again" or "Treated by irradiation-do not irradiate again" To ensure that the food is not repeatedly irradiated.
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net


