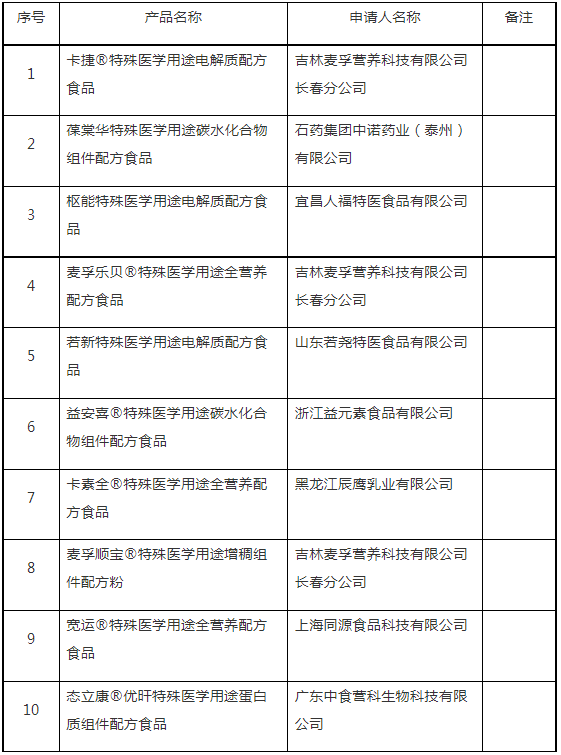
On September 1, the Center of Food evaluation of the State Administration for Market Regulation updated the list of approval document (decision) for the registration of Formula Foods for Special Medical Purpose, which involves Shuneng Electrolyte Formula Food for Special Medical Purposes, Baotanghua Carbohydrate Component Formula Food for Special Medical Purposes and other 8 products.
国家市场监督管理总局食品审评中心发布2021年08月31日和09月1日特殊医学用途配方食品注册批件(决定书)待领取信息,包含葆棠华特殊医学用途碳水化合物组件配方食品、枢能特殊医学用途电解质配方食品等10种产品。