

According to the National food safety standard of Health foods (GB 16740), health foods are defined as the food which claims to have specific healthcare functions or to be a supplement of vitamins and minerals for the human body. Health food is designed for the specific group of people, aiming to benefit the functioning of the human body whilst not treat or cure any disease. Health food shall not bring acute, sub-acute or chronic harm to the human body.
According to the "Food Safety Law of the People's Republic of China" and the "Measures for the Administration of the Registration and Recordation of Health Food", China has strict supervision and management of speciality products such as health food, and imported health food should be registered or filed with the State Administration of Market Regulation (SAMR). only after obtaining the registration certificate or filing certificate will it be marketed and sold in China.
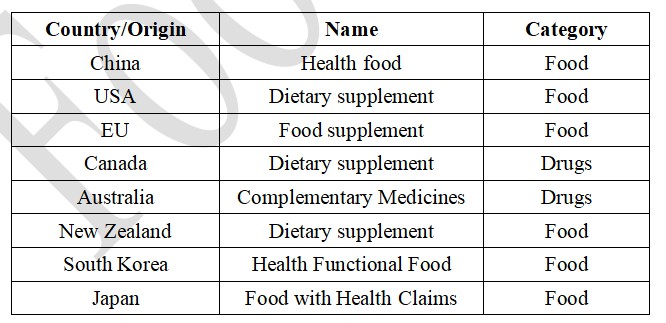
Table 1 The names of health food products in different countries
There are two systems for registering and filing health food products for import into China. The main differentiation is based on the raw materials used.
Registration Raw Materials Scope
(1) Health food that use raw materials other than those included in list of raw materials for health food (hereinafter referred to as the “non-list raw material”); or (2) Health food imported for the first time (excluding those that belong to vitamin supplements, minerals and other nutritious substances). Health food imported for the first time shall refer to health food under application for being sold on the domestic market, which are not of the same country, not of the same enterprise or not of the same formula.
Filing Raw Materials Scope
Health food products that are supplemented with vitamins, minerals and other nutrients. The nutrients shall be those listed in the list of raw materials for health food. It should be noted that the list of raw materials for health food includes not only vitamins and minerals but also other substances. Domestic health foods that use broken ganoderma lucidum spore powder, coenzyme Q10, melatonin, fish oil or spirulina as the single raw material can apply for filing in China (domestic products only). However, for imported health food products, those containing these substances do not use the filing route and need to be registered.
Claim Health Functions
Registration System:
Health food under the registration system can claim 27 health functions according to the nature of the product. The 27 functions include enhancing immune systems, antioxidative function, assisting memory improvement, alleviating eye fatigue, improving throat function, sleep improvement, alleviating physical fatigue, enhancing anoxia endurance, assisting weigh control, increasing bone density, improving nutritional anemia, eliminating acne, eliminating melasma, improving skin ability to retain moisture, regulating gastrointestinal flora, facilitation digestion, facilitating bowel movement, protection of gastric mucosa, assisting blood lipids reduction, assisting blood sugar reduction, assisting blood pressure reduction, assisting liver protection against chemical injury, irradiation hazard protection function, alleviating lead excretion, facilitating milk secretion (proposed to cancel), improving growth (proposed to cancel), improving shin oil content function (proposed to cancel).
Filing System:
Health food under the filing system can claim "vitamin and mineral supplement".
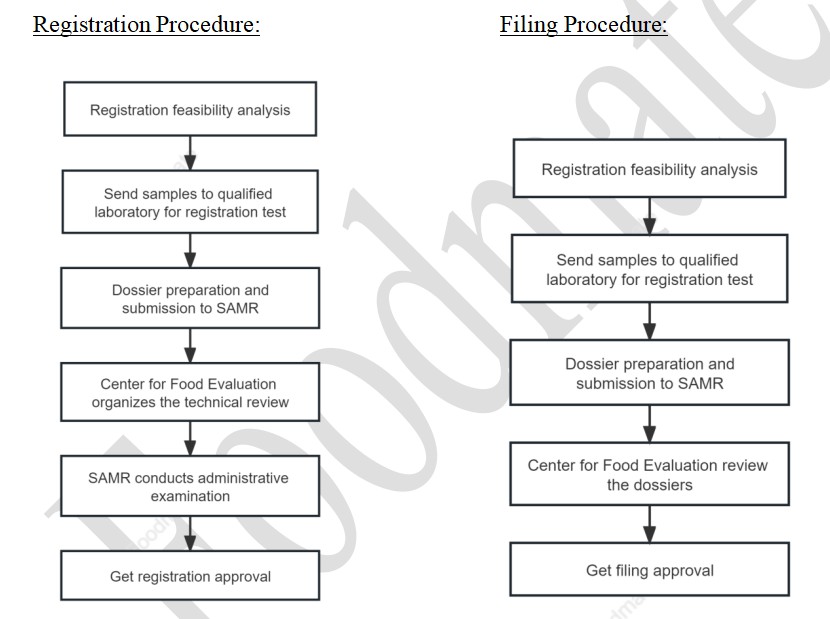
Registration/Filling Procedure
Foodmate can provide you with professional consultation services on health foods export to China, including health food registration and filing, ingredients revies, label review, etc. If you need any help, please contact us via global_info@foodmate.net.
Need help or have a question?
Send mail