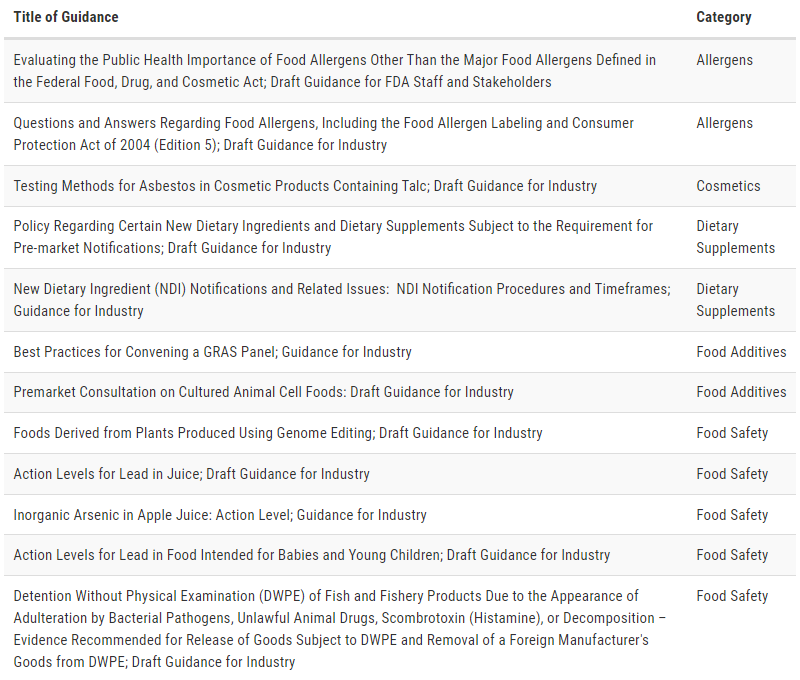
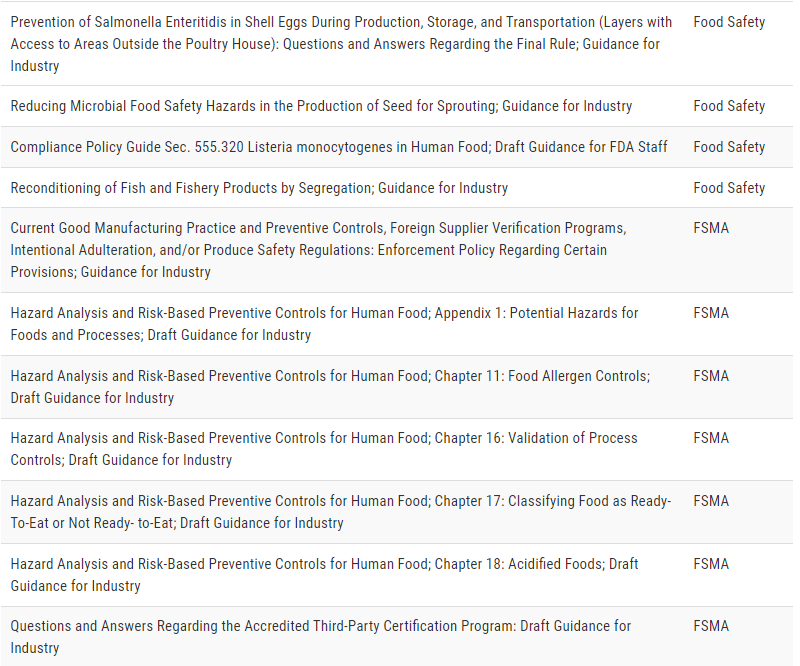
On January 31, 2022, the U.S. Food and Drug Administration’s Center for Food Safety and Applied Nutrition (CFSAN) and Office of Food Policy and Response (OFPR) released a list of draft and final guidance topics that are a priority for the FDA Foods Program to complete during the next 12 months.
The agency anticipates it will publish many of these documents by January 2023. The list is an update on the guidance agenda released in June 2021 and focuses on Level 1 draft and final guidances.
The FDA is taking this action to provide continued transparency for stakeholders regarding foods program priorities. Guidance documents represent the FDA’s current thinking on a specific topic and the information can help stakeholders plan for potential changes that may impact their businesses and organizations. They do not impose legally enforceable requirements.
Although the FDA's intent is to publish all draft and final guidance topics on the list, modifications in plans may be needed to support emerging issues and Administration priorities.