The FDA, along with CDC and state and local partners are investigating four consumer complaints of infant illness related to products from Abbott Nutrition’s Sturgis, MI facility received from 9/20/2021 to 1/11/2022. All of the cases are reported to have consumed powdered infant formula (IF) produced from Abbott Nutrition’s Sturgis, MI facility. These complaints include three reports of Cronobacter sakazakii infections and one report of Salmonella Newport infection in infants. All four cases related to these complaints were hospitalized and Cronobacter may have contributed to a death in one case.
FDA has initiated an onsite inspection at the facility. Findings to date include several positive Cronobacter results from environmental samples taken by FDA, and adverse inspectional observations by FDA investigators. A review of the firm’s internal records also indicate environmental contamination with Cronobacter sakazakii and the firm’s destruction of product due to the presence of Cronobacter.
FDA is issuing this advisory to alert consumers to avoid purchasing or using recalled powdered infant formula produced in the Sturgis, MI facility.
On 2/17/2022, Abbott Nutrition initiated a voluntary recall of certain powdered infant formulas. Products made at the Sturgis facility can be found across the United States and were likely exported to other countries as well. Canadian health officials have also issued a recall warning External link Disclaimer. FDA is continuing to investigate and will update this advisory should additional consumer safety information become available.
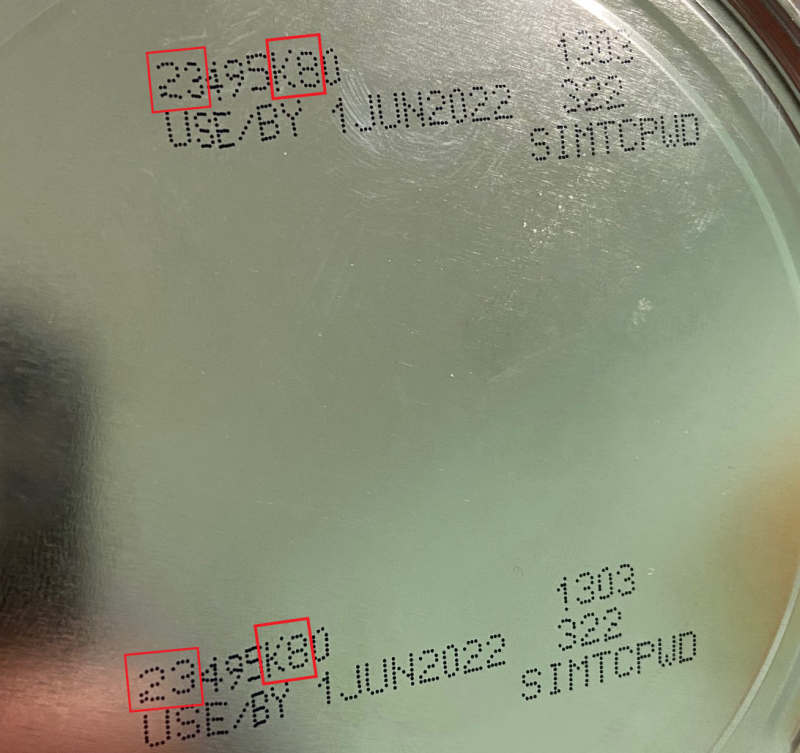
Product Images
International Product Distribution
According to the firm, recalled products were distributed to the following countries: Australia, Bahrain, Barbados, Bermuda, Canada, Chile, China, Colombia, Costa Rica, Dominican Republic, Ecuador, Egypt, Guam, Guatemala, Hong Kong, India, Indonesia, Israel, Jordan, Kuwait, Lebanon, Malaysia, Mexico, New Zealand, Oman, Peru, Puerto Rico, Qatar, Saudi Arabia, Singapore, South Africa, Sudan, Taiwan, Thailand, United Arab Emirates, United Kingdom, and Vietnam ANI South.