According to the Official Journal of the EU, on 12 August 2022, the European Commission issued (EU) Regulation 202/1393 amending the maximum residue limits for Delta-9-tetrahydrocannabinol in hemp seeds and its products. Specific amendments are as follows:
In Section 8 of the Annex to Regulation (EC) 1881/2006, add subsection 8.6:
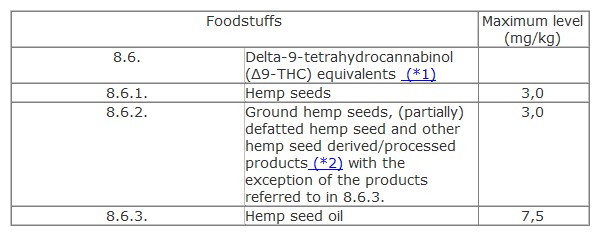
(*1) the maximum level refers to the sum of delta-9-tetrahydrocannabinol (Δ9-THC) and delta-9-tetrahydrocannabinolic acid (Δ9-THCA), expressed as Δ9-THC. A factor of 0,877 is applied to the level of Δ9-THCA and the maximum level refers to the sum of Δ9-THC + 0,877 x Δ9-THCA (in case of a separate determination and quantification of Δ9-THC and Δ9-THCA).
(*2) hemp seed derived/processed products are products derived/processed exclusively from hemp seeds.
For more details, see
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2022.211.01.0083.01.ENG&toc=OJ%3AL%3A2022%3A211%3ATOC



